Non-invasive longitudinal monitoring of vascularization in tissue-engineered grafts by ultrasound localization microscopy
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
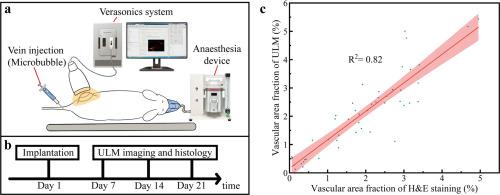
Tissue engineering is a rapidly advancing field aimed at regenerating damaged tissues by combining cells, biomaterials, and bioactive molecules. Adequate vascularization is a critical determinant of successful tissue regeneration, as it supports both the regenerative process and the maintenance of physiological functions in the newly formed tissue. Traditional methods for evaluating vascularization, such as histological and immunohistochemical techniques, are inherently invasive and limited to endpoint analysis. In this study, we innovatively explored the potential of ultrasound localization microscopy (ULM) as a non-invasive, high-resolution, and longitudinal imaging tool for monitoring vascularization in tissue-engineered grafts. Three representative types of tissue-engineered grafts, Hydrogel, Hydrogel with stem cells, and Hydrogel with growth factors were subcutaneously implanted in mice. ULM imaging was performed at multiple time points (7, 14, and 21 days post-implantation) to evaluate vascular growth within the implants dynamically. The vascularization of these grafts was further validated using conventional H&E staining and CD31 immunohistochemical staining. Our findings demonstrated that ULM had a strong correlation (R2 = 0.82 and 0.91, respectively) with conventional histological methods, confirming its accuracy as a non-invasive tool for vascular assessment in tissue engineering research.
Statement of significance
Adequate vascularization is fundamental for successful tissue regeneration, but traditional methods like histology are invasive and limited to endpoint analysis. This study innovatively utilizes ultrasound localization microscopy (ULM) as a non-invasive, high-resolution tool for longitudinal monitoring of vascularization in three tissue-engineered grafts: Hydrogel, Hydrogel with stem cells, and Hydrogel with growth factors. ULM showed a strong correlation (R2 = 0.82 and 0.91, respectively) with histological assessments (H&E staining and CD31 staining) across all time points. Notably, this represents the first demonstration of ULM for non-invasive, dynamic tracking of graft vascularization. With its deep tissue penetration and robust reliability, ULM emerges as a promising alternative for evaluating vascularization in tissue regeneration research, enabling continuous, high-precision analysis without tissue excision.
超声定位显微镜对组织工程移植物血管化的无创纵向监测。
组织工程是一个快速发展的领域,旨在通过结合细胞、生物材料和生物活性分子来再生受损组织。充分的血管化是组织再生成功的关键决定因素,因为它支持再生过程和维持新形成组织的生理功能。评估血管化的传统方法,如组织学和免疫组织化学技术,本质上是侵入性的,并且仅限于终点分析。在这项研究中,我们创新性地探索了超声定位显微镜(ULM)作为一种无创、高分辨率和纵向成像工具监测组织工程移植物血管化的潜力。将三种具有代表性的组织工程移植物:水凝胶、带干细胞的水凝胶和带生长因子的水凝胶皮下植入小鼠。在多个时间点(种植后7、14和21天)进行ULM成像,动态评估种植体内的血管生长情况。采用常规H&E染色和CD31免疫组化染色进一步验证移植物的血管化。我们的研究结果表明,ULM与常规组织学方法具有很强的相关性(R2 = 分别为0.82和0.91),证实了其作为组织工程研究中血管评估的非侵入性工具的准确性。意义声明:充分的血管化是成功组织再生的基础,但传统的方法,如组织学,是侵入性的,仅限于终点分析。本研究创新性地利用超声定位显微镜(ULM)作为一种无创、高分辨率的工具,对三种组织工程移植物(水凝胶、干细胞水凝胶和生长因子水凝胶)的血管化进行纵向监测。在所有时间点,ULM与组织学评估(H&E染色和CD31染色)有很强的相关性(R2 = ,分别为0.82和0.91)。值得注意的是,这是首次证明ULM可以无创、动态地跟踪移植物血管形成。ULM具有深入组织渗透和强大的可靠性,成为组织再生研究中评估血管形成的有希望的替代方法,可以在不切除组织的情况下进行连续、高精度的分析。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


