Substrate stiffness modulates osteogenic differentiation of BMMSCs via the hedgehog signaling pathway
IF 2.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biochemistry & Cell Biology
Pub Date : 2025-07-23
DOI:10.1016/j.biocel.2025.106840
引用次数: 0
Abstract
Substrate stiffness is a critical biophysical cue regulating mesenchymal stem cell (MSC) fate, yet the underlying mechanisms remain incompletely understood. Here, we investigated how substrate stiffness modulates the osteogenic differentiation of bone marrow mesenchymal stem cells (BMMSCs) and the involvement of the Hedgehog (Hh) signaling pathway in this process. Polydimethylsiloxane (PDMS) substrates with tunable stiffness (soft: 32.73 ± 3.74 kPa; medium: 57.59 ± 5.65 kPa; stiff: 147.4 ± 11.04 kPa) were fabricated and functionalized with arginine-glycine-aspartic acid (RGD) peptides to mimic the mechanical microenvironment of bone tissue. BMMSCs cultured on stiff substrates exhibited enhanced cell spreading and proliferation compared to those on soft substrates. Osteogenic induction experiments revealed that stiff substrates significantly upregulated alkaline phosphatase (ALP) expression and calcium nodule formation after 7 and 21 days, respectively. Mechanistically, the Hh pathway was activated on stiff substrates at day 3. Inhibition of Hh signaling using GANT61 impeded stiffness-induced effects, reducing cell spreading, proliferation, and osteogenic differentiation. These findings demonstrate that substrate stiffness promotes BMMSCs osteogenesis in a Hh signaling-dependent manner, providing new insights into the mechanobiology of bone regeneration and informing the design of stiffness-optimized biomaterials for tissue engineering applications.
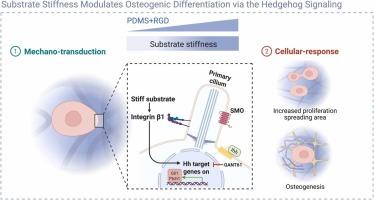
底物硬度通过Hedgehog信号通路调节BMMSCs的成骨分化。
基质硬度是调节间充质干细胞(MSC)命运的关键生物物理线索,但其潜在机制尚不完全清楚。在这里,我们研究了底物硬度如何调节骨髓间充质干细胞(BMMSCs)的成骨分化以及Hedgehog (Hh)信号通路在这一过程中的参与。硬度可调的聚二甲基硅氧烷(PDMS)衬底(软:32.73±3.74 kPa;介质:57.59±5.65 kPa;采用精氨酸-甘氨酸-天冬氨酸(RGD)多肽对其进行功能化,模拟骨组织的机械微环境。与在软基质上培养的骨髓间充质干细胞相比,在硬基质上培养的骨髓间充质干细胞表现出更强的细胞扩散和增殖。成骨诱导实验显示,在7天和21天后,硬底物分别显著上调碱性磷酸酶(ALP)的表达和钙结节的形成。机械上,Hh通路在第3天在坚硬底物上被激活。使用GANT61抑制Hh信号会阻碍僵硬诱导的效应,减少细胞扩散、增殖和成骨分化。这些发现表明,基质刚度以Hh信号依赖的方式促进BMMSCs成骨,为骨再生的机械生物学提供了新的见解,并为组织工程应用中刚度优化的生物材料的设计提供了信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.10
自引率
0.00%
发文量
124
审稿时长
19 days
期刊介绍:
IJBCB publishes original research articles, invited reviews and in-focus articles in all areas of cell and molecular biology and biomedical research.
Topics of interest include, but are not limited to:
-Mechanistic studies of cells, cell organelles, sub-cellular molecular pathways and metabolism
-Novel insights into disease pathogenesis
-Nanotechnology with implication to biological and medical processes
-Genomics and bioinformatics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


