Gut microbiota-related glutathione metabolism is key mechanism for sulforaphane ameliorating ulcerative colitis
IF 4.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Gut barrier dysfunction is associated with dysbiosis of the gut microbiota and its metabolites, which is closely linked to the pathogenesis of ulcerative colitis (UC). Recent studies have demonstrated that Sulforaphane (SFN) exerts beneficial effects on UC. However, the role of the gut microbiota and microbial metabolism in the anti-UC mechanisms of SFN remains inadequately understood. In this study, we observed that SFN administration significantly improved the pathological phenotype, restored gut barrier integrity, and reduced colon inflammation in dextran sulfate sodium (DSS)-induced colitis mice. Gut microbiota analysis illustrated that SFN administration rebalances the alterations in gut microbiota composition, including genera such as Turicibacter, Lactobacillus and Bacteroides, in DSS-induced mice. Furthermore, untargeted metabolomics analysis indicated that the levels of microbial arachidonic acid metabolism, as well as the metabolism of alanine, aspartate, and glutamate, and glutathione metabolism in the gastrointestinal tract, were significantly altered in DSS-induced mice. Interestingly, SFN treatment significantly restore the alterations in glutathione metabolism and the levels of associated metabolites. Additionally, we observed that the MAPK/NF-κB signaling pathway, regulated by glutathione metabolism, was inhibited in the colon of DSS-induced mice following SFN treatment. Collectively, these results suggest that SFN can alleviate DSS-induced colitis in mice by restoring dysregulated gut microbiota and glutathione metabolism, thereby modulating the MAPK/NF-κB signaling pathway, enhancing intestinal barrier function and reducing colonic inflammation. Importantly, our findings elucidate a novel mechanism by which SFN improves gut barrier function, highlighting its potential to advance the development of SFN-derived therapeutics for the clinical management of colitis.
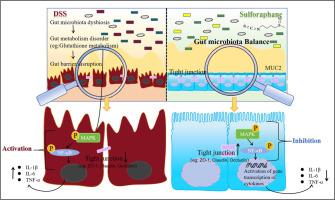
肠道菌群相关谷胱甘肽代谢是萝卜硫素改善溃疡性结肠炎的关键机制。
肠道屏障功能障碍与肠道菌群及其代谢物的生态失调有关,这与溃疡性结肠炎(UC)的发病机制密切相关。最近的研究表明,萝卜硫素(SFN)对UC有有益的作用。然而,肠道微生物群和微生物代谢在SFN抗uc机制中的作用仍未充分了解。在本研究中,我们观察到SFN给药显著改善了葡聚糖硫酸钠(DSS)诱导的结肠炎小鼠的病理表型,恢复了肠道屏障的完整性,并减少了结肠炎症。肠道菌群分析表明,SFN重新平衡了dss诱导小鼠肠道菌群组成的变化,包括Turicibacter, Lactobacillus和Bacteroides等属。此外,非靶向代谢组学分析表明,dss诱导小鼠胃肠道微生物花生四烯酸代谢水平、丙氨酸、天冬氨酸、谷氨酸代谢和谷胱甘肽代谢水平显著改变。有趣的是,SFN治疗显著恢复了谷胱甘肽代谢和相关代谢物水平的改变。此外,我们观察到SFN处理后dss诱导小鼠结肠中受谷胱甘肽代谢调节的MAPK/NF-κB信号通路被抑制。综上所述,SFN可通过恢复失调的肠道菌群和谷胱甘肽代谢,从而调节MAPK/NF-κB信号通路,增强肠道屏障功能,减轻结肠炎症,从而减轻dss诱导的小鼠结肠炎。重要的是,我们的研究结果阐明了SFN改善肠道屏障功能的新机制,强调了其促进SFN衍生治疗方法用于结肠炎临床管理的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


