Molecular profiling of the Australian plague locust pathobiome reveals a microbial driver of population suppression
IF 2.4
3区 生物学
Q1 ZOOLOGY
引用次数: 0
Abstract
Despite recent advances in surveillance, forecasting, and management, locust plagues remain a chronic threat to global agriculture. Part of the intractability of this issue lies in the complex interplay of ecological factors driving locust population dynamics, of which the locust microbiome remains an understudied–but crucial–component. During the wet Australian summers of 2020-2022, forecasting models predicted that Australian plague locust (Chortoicetes terminifera) populations would reach plague proportions, while field-surveillance revealed the contrary. To investigate a putative microbiological cause for this population suppression, a diagnostic framework integrating metabarcoding, microbial isolation, genomic characterisation, and pathogenicity assays was applied. This approach identified a bacterial pathogen belonging to the Serratia marcescens species complex and demonstrated its entomopathogenicity through controlled feeding assays. Genome mining of the isolated S. marcescens strain revealed virulence factors associated with insecticidal activity, including urease-mediated cytotoxicity and metal-scavenging biosynthetic gene clusters. Finally, field population screening indicated that S. marcescens presence was not uniform across surveyed APL populations, suggesting that infections may be transient or epidemic in nature, potentially influenced by environmental conditions such as temperature and humidity. These findings stress the importance of microbial interactions in shaping locust population dynamics and establish a basis for future research into ecological strategies for locust control.
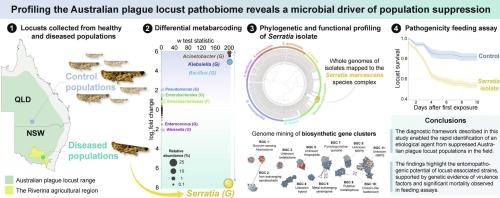
澳大利亚蝗灾病原组的分子分析揭示了种群抑制的微生物驱动因素。
尽管最近在监测、预报和管理方面取得了进展,但蝗灾仍然是全球农业的长期威胁。这个问题的难处部分在于驱动蝗虫种群动态的生态因素之间复杂的相互作用,其中蝗虫微生物群仍然是一个研究不足但至关重要的组成部分。在2020-2022年澳大利亚潮湿的夏季,预测模型预测澳大利亚鼠疫蝗(Chortoicetes terminifera)的种群数量将达到鼠疫的比例,而现场监测显示相反。为了研究这种种群抑制的假定微生物原因,应用了整合元条形码、微生物分离、基因组特征和致病性分析的诊断框架。该方法鉴定了粘质沙雷氏菌属的一种细菌病原体,并通过控制饲养试验证明了其昆虫致病性。对分离的粘质葡萄球菌菌株的基因组挖掘揭示了与杀虫活性相关的毒力因子,包括脲酶介导的细胞毒性和清除金属的生物合成基因簇。最后,现场种群筛选表明,在调查的APL种群中,粘质葡萄球菌的存在并不均匀,这表明感染可能是短暂的或流行的,可能受到温度和湿度等环境条件的影响。这些发现强调了微生物相互作用在形成蝗虫种群动态方面的重要性,并为今后研究蝗虫控制的生态策略奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.10
自引率
5.90%
发文量
94
审稿时长
1 months
期刊介绍:
The Journal of Invertebrate Pathology presents original research articles and notes on the induction and pathogenesis of diseases of invertebrates, including the suppression of diseases in beneficial species, and the use of diseases in controlling undesirable species. In addition, the journal publishes the results of physiological, morphological, genetic, immunological and ecological studies as related to the etiologic agents of diseases of invertebrates.
The Journal of Invertebrate Pathology is the adopted journal of the Society for Invertebrate Pathology, and is available to SIP members at a special reduced price.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


