Compromised repolarization reserve in a murine model of catecholaminergic polymorphic ventricular tachycardia caused by RyR2-R420Q mutation
IF 4.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a malignant inherited heart disease characterised by stress-induced arrhythmias that are thought to be caused by delayed afterdepolarizations resulting from abnormal Ca2+ cycling. Some patients exhibit unusually large ECG U-waves that could be associated with altered ventricular repolarization, but the possible link with dysfunctional RyR2 is unclear. We investigated whether increased Ca2+ leak during systole disrupts repolarization in a transgenic mouse model of CPVT.
Methods
Electrocardiograms were recorded in patients with RyR2-R420Q CPVT mutation (R420Q). Experiments were performed on control and R420Q knock-in mouse hearts and ventricular myocytes.
Results
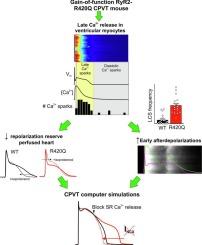
R420Q patients had larger resting U-waves than family member controls. R420Q mouse hearts exhibited greater prolongation of monophasic APs following pauses in pacing and during beta-adrenergic stimulation. Ventricular ectopic beats during repolarization were more prevalent in R420Q mouse hearts following pacing-pauses and during premature electrical stimulation. Early afterdepolarizations (EADs) occurred in isolated R420Q myocytes during beta-adrenergic stimulation and coincided with increased Ca2+ leak during the Ca2+ transient decay, in the form of late Ca2+ sparks (LCS). AP voltage clamp electrophysiology experiments, analysis of LCS recovery, and computer simulations of hyperactive RyR2 supported a mechanism involving increased RyR2 sensitivity and/or reduced refractoriness that increased LCS frequency and inward sodium/calcium exchange current, resulting in AP prolongation and EADs.
Conclusions
Ca2+-mediated AP lengthening and EADs may contribute to proarrhythmic behaviour in CPVT caused by gain-of-function R420Q mutation. Loss of repolarization reserve is not specifically targeted by CPVT therapies but could be an opportunity for therapeutic intervention.
RyR2-R420Q突变致儿茶酚胺能多态性室性心动过速小鼠模型复极化储备受损
背景:儿茶酚胺能多形性室性心动过速(CPVT)是一种以应激性心律失常为特征的恶性遗传性心脏病,被认为是由Ca2+异常循环导致的后去极化延迟引起的。一些患者表现出异常大的心电图u波,这可能与心室复极改变有关,但与RyR2功能障碍的可能联系尚不清楚。我们研究了在CPVT转基因小鼠模型中,收缩期Ca2+泄漏增加是否会破坏复极化。方法:记录RyR2-R420Q CPVT突变(R420Q)患者的心电图。实验在对照组和R420Q敲入小鼠心脏和心室肌细胞上进行。结果:R420Q患者的静息u波大于家庭成员对照组。R420Q小鼠心脏在起搏暂停和β -肾上腺素能刺激期间表现出更长的单相ap。R420Q小鼠心脏在起搏暂停和过早电刺激时,复极期间的室性异搏更为普遍。早期后去极化(EADs)发生在分离的R420Q肌细胞在β -肾上腺素能刺激期间,并与Ca2+瞬态衰减期间增加的Ca2+泄漏相吻合,以晚期Ca2+火花(LCS)的形式。AP电压钳电生理实验、LCS恢复分析以及过度活跃的RyR2的计算机模拟支持了一种机制,该机制涉及RyR2敏感性增加和/或耐受性降低,从而增加LCS频率和向内钠/钙交换电流,导致AP延长和EADs。结论:Ca2+介导的AP延长和EADs可能有助于功能获得性R420Q突变引起的CPVT的心律失常行为。复极储备的丧失不是CPVT治疗的专门目标,但可能是治疗干预的机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


