Astrocyte specification in the mouse septum is shaped by both developmental origin and local signals
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
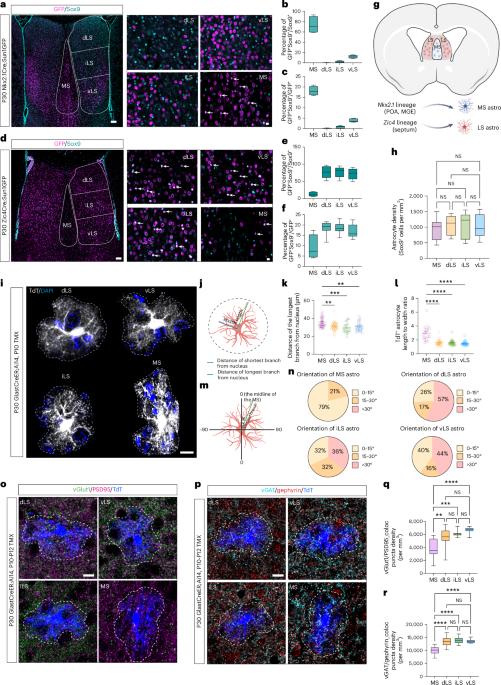
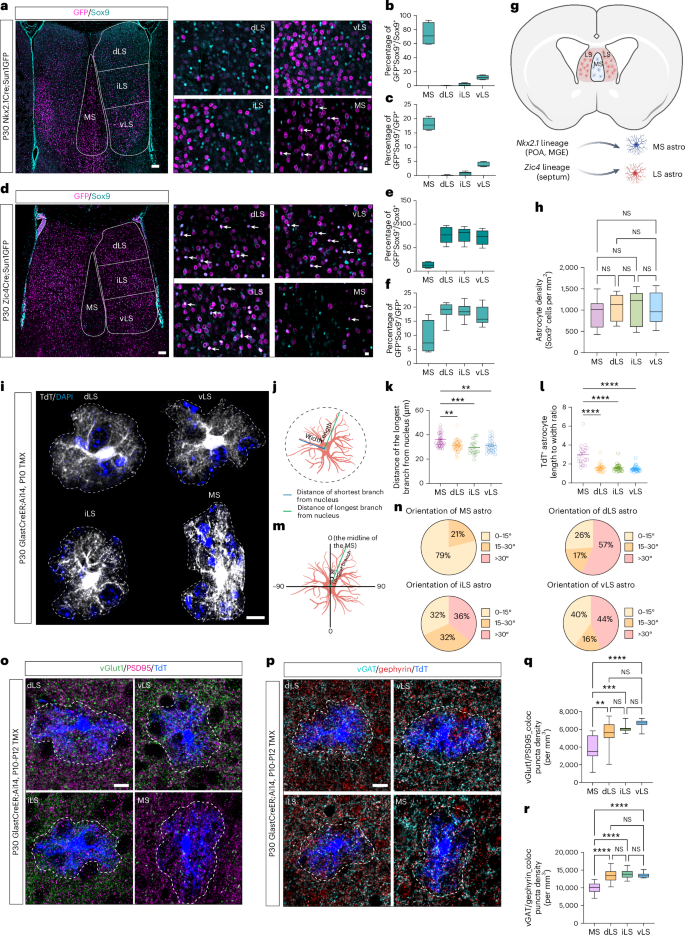
Astrocyte specification during development is influenced by both intrinsic and extrinsic factors, but the precise contribution of each remains poorly understood. Here we show that mouse septal astrocytes derived from Nkx2.1- and Zic4-expressing progenitor zones are primarily allocated into the medial septal and lateral septal nuclei, respectively. Astrocytes in these areas exhibit distinctive molecular and morphological features. Using single-nucleus RNA sequencing, we traced the developmental trajectories of cells in the septum and found that neurons and astrocytes undergo region-specific and developmental-stage-specific local cell–cell interactions. Expression of the morphogens sonic hedgehog and fibroblast growth factors by medial septal and lateral septal neurons, respectively, promote the specification of astrocytes in each region. Finally, heterotopic cell transplantation studies showed that septal astrocyte specification depends on the local microenvironment, regardless of developmental origin. Our data highlight the importance of the local environment in determining astrocyte functional specialization. The relative contribution of intrinsic and extrinsic factors to astrocyte heterogeneity is unknown. Using heterotropic transplantation of septal astrocytes with distinct lineage origins, we show that local factors specify their ultimate identities.
小鼠鼻中隔的星形胶质细胞规格是由发育起源和局部信号共同决定的
星形胶质细胞在发育过程中受到内在和外在因素的影响,但每种因素的确切作用仍然知之甚少。本研究表明,来自表达Nkx2.1-和zic4祖细胞区的小鼠间隔星形胶质细胞主要分别分布于间隔内侧核和间隔外侧核。这些区域的星形胶质细胞表现出独特的分子和形态特征。利用单核RNA测序,我们追踪了中隔细胞的发育轨迹,发现神经元和星形胶质细胞经历了区域特异性和发育阶段特异性的局部细胞-细胞相互作用。内间隔和外侧间隔神经元分别表达形态因子sonic hedgehog和成纤维细胞生长因子,促进各区域星形胶质细胞的特化。最后,异位细胞移植研究表明,鼻中隔星形胶质细胞规格取决于局部微环境,而与发育起源无关。我们的数据强调了局部环境在决定星形胶质细胞功能特化中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


