The synaptic architecture of layer 5 thick tufted excitatory neurons in mouse visual cortex
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
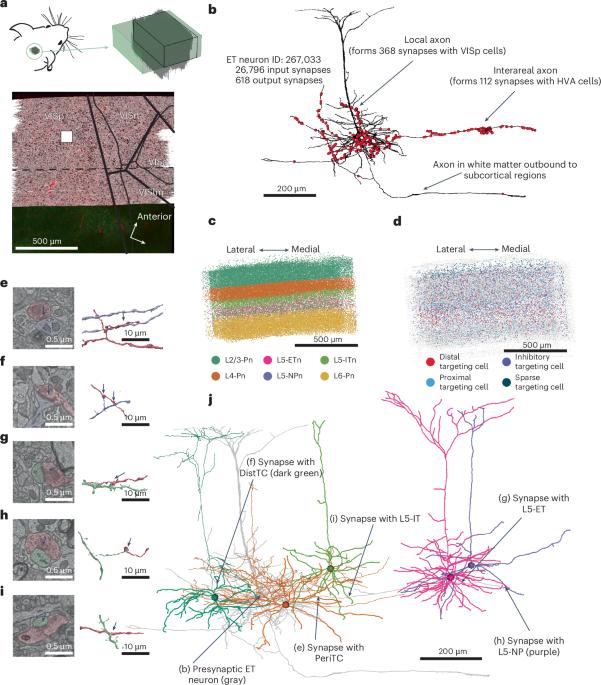
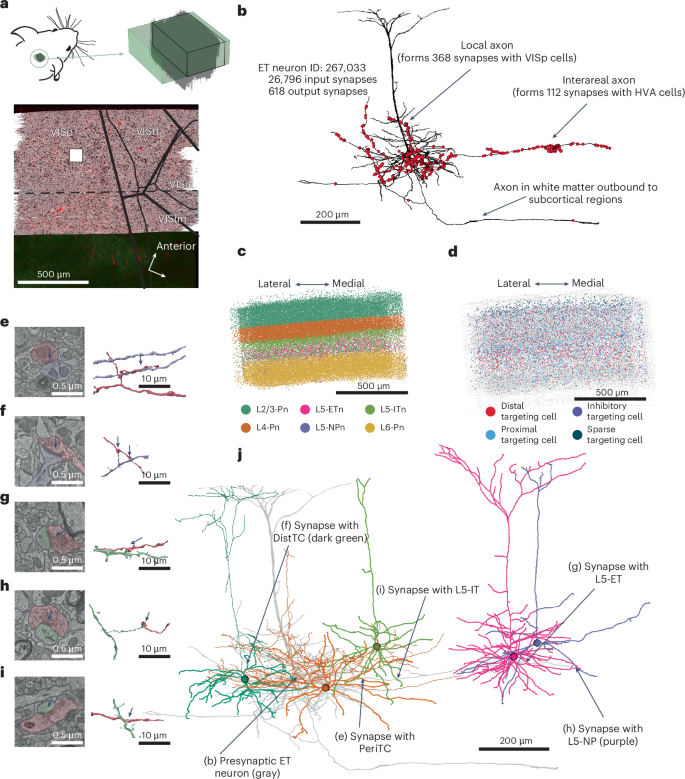
Despite significant progress in characterizing neocortical cell types, a complete understanding of the synaptic connections of individual excitatory cells remains elusive. This study investigates the connectivity of mouse visual cortex thick tufted layer 5 pyramidal cells, also known as extratelencephalic neurons (L5-ETns), using a 1 mm3 publicly available electron microscopy dataset. The analysis reveals that, in their immediate vicinity, L5-ETns primarily establish connections with a group of inhibitory cell types, which, in turn, specifically target the L5-ETns back. The most common excitatory targets of L5-ETns are layer 5 intertelencephalic neurons (L5-ITns) and layer 6 (L6) pyramidal cells, whereas synapses with other L5-ETns are less common. When L5-ETns extend their axons to other cortical regions, they tend to connect more with excitatory cells. Our results highlight a circuit motif where a subclass of excitatory cells forms a subcircuit with specific inhibitory cell types. This is achieved using a publicly available, automated approach for synapse recognition and automated cell typing, offering a framework for exploring the connectivity of other neuron types. This study maps the connections of layer 5 pyramidal neurons in the mouse cortex, revealing distinct local and intercortical wiring patterns, and provides an open framework for exploring the connectivity of cell types.
小鼠视觉皮层第5层厚丛状兴奋性神经元的突触结构
尽管在表征新皮质细胞类型方面取得了重大进展,但对单个兴奋性细胞的突触连接的完整理解仍然难以捉摸。本研究使用1 mm3公开的电子显微镜数据集研究了小鼠视觉皮层厚簇状第5层锥体细胞(也称为脑外神经元(L5-ETns))的连通性。分析表明,在L5-ETns的邻近区域,L5-ETns主要与一组抑制性细胞类型建立联系,而抑制性细胞类型反过来又专门针对L5-ETns。L5-ETns最常见的兴奋靶点是第5层脑间神经元(L5-ITns)和第6层锥体细胞,而与其他L5-ETns的突触则不太常见。当l5 - etn将轴突延伸到其他皮质区域时,它们倾向于更多地与兴奋性细胞连接。我们的研究结果突出了一个电路基序,其中一个亚类兴奋性细胞与特定的抑制性细胞类型形成一个亚回路。这是通过公开可用的自动突触识别和自动细胞分型方法实现的,为探索其他神经元类型的连通性提供了一个框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


