Gut dysbiosis influences the pathophysiology of multiple sclerosis: A case-control study from North India
IF 2.5
4区 医学
Q3 IMMUNOLOGY
引用次数: 0
Abstract
Background and objectives
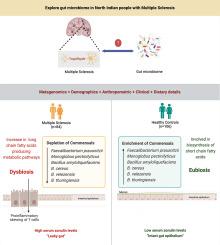
Alterations in gut microbiota have been linked to pathophysiology of immune-mediated diseases like multiple sclerosis (MS). This study was undertaken to characterise the gut microbiome profile in North Indian MS patients and to evaluate gut health using biomarkers like zonulin (intestinal permeability) and calprotectin (intestinal inflammation).
Methods
84 Patients with relapsing-remitting MS patients (RRMS) of 18–75 years of age with an expanded disability status scale (EDSS) score less than or equal to 5.5 and 106 healthy controls (HC) were recruited for the study. Gut microbiota was sequenced using Illumina MiSeq. Clinical, demographic, anthropometric, and dietary details were recorded. Sandwich ELISA was used to quantify serum zonulin and fecal calprotectin levels.
Results
MS patients had lower alpha microbial diversity, while distinct beta diversity metrics were observed in MS and HC. Firmicutes was found to be the most abundant phylum in both groups with significant enrichment in MS than HC. In MS, significant depletion of commensal bacterial species like Faecalibacterium prausnitzii, Monoglobus pectinilyticus, and Bacillus species indicated gut dysbiosis. These alterations influenced the prevalence and functioning of metabolic pathways. Therefore, pathways involved in biosynthesis of long-chain fatty acids (LCFA) were significantly enriched in MS than HC, while generation of short-chain fatty acids were predominant in HC. In addition, high zonulin, without an increase in calprotectin levels was observed in MS patients.
Conclusions
RRMS patients in North India have a decreased microbial diversity in terms of depletion of commensals. The dominance of LCFA generating pathways in MS patients might have triggered the proinflammatory reactions, that are possibly linked to the development of a highly permeable/leaky gut in MS.
肠道失调影响多发性硬化症的病理生理:一项来自北印度的病例对照研究
背景和目的肠道微生物群的改变与免疫介导的疾病如多发性硬化症(MS)的病理生理有关。本研究旨在描述北印度MS患者的肠道微生物群特征,并使用zonulin(肠道通透性)和钙保护蛋白(肠道炎症)等生物标志物评估肠道健康。方法84例18-75岁扩展残疾状态量表(EDSS)评分小于或等于5.5分的复发-缓解型MS患者(RRMS)和106例健康对照(HC)。使用Illumina MiSeq对肠道菌群进行测序。记录临床、人口统计学、人体测量学和饮食细节。采用夹心ELISA法定量测定血清zonulin和粪便钙保护蛋白水平。结果多发性硬化症患者的α微生物多样性较低,而多发性硬化症和HC患者的β微生物多样性指标不同。厚壁菌门是两组中最丰富的门,MS比HC富集显著。在多发性硬化症中,共生细菌如prausnitzii Faecalibacterium, monglobus pectinilyticus和芽孢杆菌物种的显著减少表明肠道生态失调。这些改变影响了代谢途径的流行和功能。因此,参与长链脂肪酸(LCFA)生物合成的途径在MS中比HC显著丰富,而短链脂肪酸的生成在HC中占主导地位。此外,在MS患者中观察到高zonulin,而没有钙保护蛋白水平的增加。结论印度北部srrms患者微生物多样性下降,群落耗竭。MS患者中LCFA生成途径的优势可能引发了促炎反应,这可能与MS中高渗透性/漏性肠道的发展有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of neuroimmunology
医学-免疫学
CiteScore
6.10
自引率
3.00%
发文量
154
审稿时长
37 days
期刊介绍:
The Journal of Neuroimmunology affords a forum for the publication of works applying immunologic methodology to the furtherance of the neurological sciences. Studies on all branches of the neurosciences, particularly fundamental and applied neurobiology, neurology, neuropathology, neurochemistry, neurovirology, neuroendocrinology, neuromuscular research, neuropharmacology and psychology, which involve either immunologic methodology (e.g. immunocytochemistry) or fundamental immunology (e.g. antibody and lymphocyte assays), are considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


