Ferrous ions in enhancing chalcopyrite-molybdenite flotation separation: Insights from solution chemistry and interfacial adsorption
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
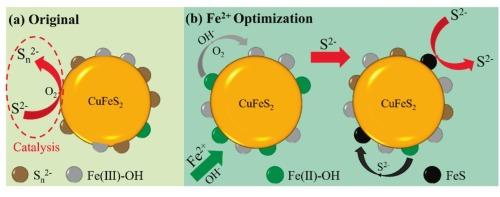
Molybdenum (Mo) is a rare metal in nature and primarily obtained from copper-molybdenum mines. Chalcopyrite (Ccp) has excellent floatability, which is usually necessary to add a large dosage of sodium sulfide (Na2S) to inhibit it. The reducing properties of Na2S make it easy to be oxidized by air, and the catalytic oxidation of sulfide surfaces will also accelerate its oxidation. Our research shows that adding ferrous (Fe2+) ions to the Na2S pulp can effectively remove the dissolved oxygen (DO) in the pulp, reduce the pulp potential, and form a hydrophilic layer of the iron sulfide, hydroxyl iron, and polysulfide on the chalcopyrite surface. This not only enhances its inhibition effect but also slows down the oxidation speed of sulfur ions and effectively enhances the utilization of Na2S. Flotation tests demonstrate that adding 3.5 kg/t ferrous sulfate synergistically reduces Na2S dosage by 3.0 kg/t (25.0 % decrease), while concurrently upgrading the concentrate grade from 3.5 % to 4.7 %, with recovery consistently maintained at 86 %. These findings not only optimize the separation process and reduce the cost of reagents but also alleviate safety and environmental concerns. This method is simple, effective, and highly applicable, offering strong practical significance for similar types of mines.
亚铁离子促进黄铜矿-辉钼矿浮选分离:来自溶液化学和界面吸附的见解
钼(Mo)是自然界的稀有金属,主要来源于铜钼矿。黄铜矿具有优良的可浮性,通常需要添加大剂量的硫化钠(Na2S)加以抑制。Na2S的还原性使其容易被空气氧化,硫化物表面的催化氧化也会加速其氧化。我们的研究表明,在Na2S矿浆中加入铁离子(Fe2+)可以有效去除矿浆中的溶解氧(DO),降低矿浆电位,并在黄铜矿表面形成硫化铁、羟基铁和多硫化物的亲水性层。这不仅增强了其抑制作用,而且减缓了硫离子的氧化速度,有效提高了Na2S的利用率。浮选试验表明,添加3.5 kg/t硫酸亚铁可协同降低Na2S用量3.0 kg/t(减少25.0 %),同时将精矿品位从3.5 %提高到4.7 %,回收率稳定保持在86 %。这些发现不仅优化了分离过程,降低了试剂成本,而且减轻了安全和环境问题。该方法简单有效,适用性强,对同类矿山具有较强的现实意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


