Age-dependent oral drug absorption in children: Assessment of a bottom-up modelling approach
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-07-22
DOI:10.1016/j.ejpb.2025.114815
引用次数: 0
Abstract
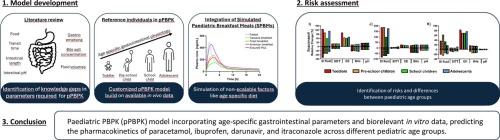
Paediatric drug development poses significant challenges due to the unique physiological and ethical considerations in children. Physiologically Based Pharmacokinetic (PBPK) modelling has emerged as a valuable tool for predicting paediatric drug absorption and optimising dosing strategies. This study aimed to refine PBPK modelling for paediatric applications by developing a customised PK-Sim-based paediatric PBPK (pPBPK) model for four drugs that are part of the Model List of Essential Medicines for Children by the World Health Organisation: paracetamol, ibuprofen, darunavir, and itraconazole. The model incorporated age-specific gastrointestinal parameters for four paediatric age groups, minimising the need for parameter interpolation or scaling. The effect of food on drug absorption was investigated by extending the absorption model to account for bile and excipient solubilisation. A literature review identified gaps in the understanding of paediatric intestinal parameters necessary for pPBPK modelling. Biorelevant in vitro dissolution and solubility data were integrated to enhance prediction accuracy. Validation with clinical pharmacokinetic data demonstrated the model’s reliability across different paediatric age groups. Sensitivity analyses highlighted the influence of gastric emptying time, small intestinal transit, and bile salt concentration on drug pharmacokinetics. This research underscores the potential of pPBPK modelling to inform paediatric dosing strategies while addressing current gaps and challenges.
儿童年龄依赖性口服药物吸收:自下而上建模方法的评估。
由于儿童独特的生理和伦理考虑,儿科药物开发面临重大挑战。基于生理的药代动力学(PBPK)模型已经成为预测儿科药物吸收和优化给药策略的有价值的工具。本研究旨在通过为世界卫生组织儿童基本药物标准清单中的四种药物(扑热息痛、布洛芬、达那韦和伊曲康唑)开发定制的基于pk - sim的儿科PBPK (pPBPK)模型,以改进PBPK模型的儿科应用。该模型纳入了四个儿科年龄组的年龄特异性胃肠道参数,最大限度地减少了参数插值或缩放的需要。通过扩展吸收模型来考虑胆汁和赋形剂的溶解,研究了食物对药物吸收的影响。一项文献综述确定了对pPBPK建模所需的儿科肠道参数的理解存在空白。整合了生物相关的体外溶出度和溶解度数据,以提高预测准确性。临床药代动力学数据验证了该模型在不同儿科年龄组的可靠性。敏感性分析强调胃排空时间、小肠运输和胆盐浓度对药物药代动力学的影响。这项研究强调了pPBPK模型在解决当前差距和挑战的同时,为儿科给药策略提供信息的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


