Decarbonative routes to generate N-acyliminium ion intermediates for the synthesis of 3-substituted isoindolinones via an intermolecular amidoalkylation reaction†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An alternative and unique approach for synthesizing 3-substituted isoindolinones via Lewis acid-catalyzed C–C bond-breaking reactions has been reported. For this purpose, we successfully generated N-acyliminium ion intermediates from two different substrates [through (a) an FeCl3 catalyzed Csp3–Csp2 bond-breaking reaction in isoindolinones bearing electron-rich and sterically bulky aryl substituents at the 3-position and (b) a ZnCl2 catalyzed Csp3–Csp3 bond-breaking reaction in isoindolinones bearing 1,3-diketo substituents at the 3-position of the isoindolinone starting precursors], which were trapped by appropriate nucleophiles to produce more stable γ-substituted isoindolinones in good to excellent yields. The leaving ability of 1,3-diketones is higher than that of electron-rich and sterically bulky arenes in our developed method, which results in higher yields of γ-substituted isoindolinones from the starting precursor bearing the 1,3-diketo substituents at its 3-position. Theoretical investigations were also carried out to gain insight into the two different C–C bond-cleaving reactions.
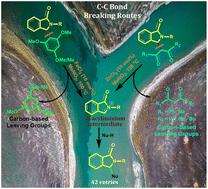
通过分子间酰胺烷基化反应制备3-取代异吲哚酮用n -酰基铵离子中间体的脱碳路线。
报道了一种利用Lewis酸催化的C-C断键反应合成3-取代异吲哚酮的独特方法。为此,我们成功地从两种不同的底物中生成了n-酰基离子中间体[通过(a) FeCl3催化的Csp3-Csp2断键反应,在异吲哚酮起始前体的3位上含有富含电子和空间体积庞大的芳基取代基的异吲哚酮中,(b) ZnCl2催化的Csp3-Csp3断键反应]。它们被合适的亲核试剂捕获,以较好的收率生产更稳定的γ-取代异吲哚酮。在我们所开发的方法中,1,3-二酮的离开能力比电子丰富且空间体积大的芳烃的离开能力高,这使得在其3位上带有1,3-二酮取代基的起始前驱体的γ-取代异吲哚酮的产率更高。还进行了理论研究,以深入了解两种不同的C-C键裂解反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


