Sensory Afferent Neural Circuits Mediate Electroacupuncture to Improve Swallowing Function in a Post-Stroke Dysphagia Mouse Model
Abstract
Background
Electroacupuncture (EA) has been reported to improve post-stroke dysphagia (PSD) effectively. However, the underlying afferent neural circuit and neurological mechanism involved in improving PSD remain poorly understood.
Methods
A PSD mouse model was established via the photochemical embolization method. Laser scatter contrast imaging was used to evaluate blood perfusion. Videofluoroscopic swallowing study, flexible endoscopic evaluation swallowing, and electromyography were used to assess the swallowing function. Neuronal activities and neuron types were detected by immunofluorescence. Synaptic connections between the nucleus tractus solitarii (NTS), the ventral posteromedial thalamic nucleus (VPM), and the primary sensory cortex (S1) were verified by neural tracing. Finally, photogenetic, chemogenetic, and in vivo electromyography or electrophysiological records were used to explore the possible afferent neural circuits of EA therapy for PSD.
Results
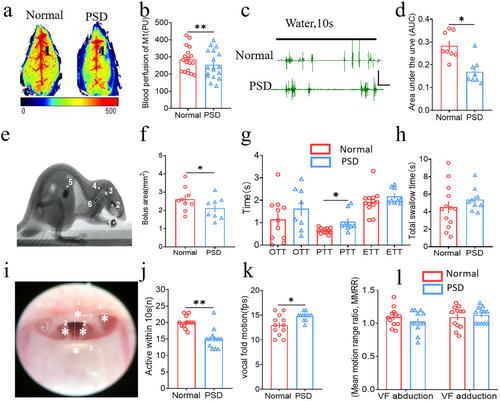
EA treatment potentiated the blood perfusion of CV23 and S1, improved the area under the curve, pharyngeal transit time, and vocal fold mobility in PSD model mice. EA also activated neuronal activities in VPM, while chemical genetic inhibition of VPM attenuated the swallowing function of EA enhanced in PSD mice. Neural tracing revealed the presence of direct synaptic connections in the neural circuit of NTS-VPM-S1, and excitatory neurons were the predominant type of synaptic projection. Activation of this circuit improved the swallowing function in PSD mice, whereas its inhibition impaired the swallowing function; this effect was reversible by EA-CV23.
Conclusion
Our findings uncover the importance of sensory afferent neural circuits NTS-VPM-S1 in driving the protective effect of EA-CV23 against dysphagia and thus reveal a potential strategy for PSD intervention.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


