Highly efficient and stable NiFe oxide-based electrocatalysts for oxygen evolution in alkaline and saline solutions
IF 8.7
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
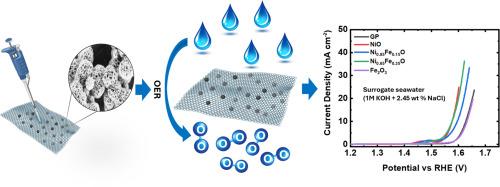
Developing cost-effective and stable oxygen evolution reaction (OER) catalysts is crucial for advancing hydrogen production via water electrolysis. Given the growing scarcity of freshwater resources, seawater electrolysis offers a promising alternative. However, maintaining both high catalytic activity and long-term durability in saline environments remains a significant challenge. In this study, four catalysts, nickel oxide (NiO), two nickel-iron oxides (Ni₀.₈₅Fe₀.₁₅O and Ni₀.₆₅Fe₀.₃₅O), and iron oxide (Fe₂O₃), were synthesized using a simple chemical bath deposition method and systematically characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), UV–visible spectroscopy, and X-ray photoelectron spectroscopy (XPS). Among them, Ni₀.₈₅Fe₀.₁₅O exhibited the best OER performance, achieving a low overpotential of 348 mV at 10 mA cm⁻² and a Tafel slope of 52 mV dec⁻¹ after 24 h of operation in surrogate seawater (1 M KOH + 2.45 wt % NaCl). This superior activity is attributed to its compact nanosheet morphology and the synergistic interaction between Ni²⁺ and Fe³⁺, which induces lattice strain and increases the density of active sites, as confirmed by SEM and XPS. Electrochemical surface area (ECSA) analysis further revealed a high number of accessible and stable active sites under saline conditions, supporting the catalyst’s intrinsic activity. Ni₀.₈₅Fe₀.₁₅O demonstrates OER performance and durability comparable to state-of-the-art seawater electrolysis catalysts, underscoring its potential for scalable and sustainable hydrogen production.
高效稳定的NiFe氧化物基电催化剂,用于碱性和盐水溶液中的析氧
开发经济、稳定的析氧反应(OER)催化剂是推进水电解制氢的关键。鉴于淡水资源日益稀缺,海水电解提供了一个有希望的替代方案。然而,在盐水环境中保持高催化活性和长期耐久性仍然是一个重大挑战。在本研究中,四种催化剂,氧化镍(NiO),两种镍铁氧化物(Ni 0 .₈₅Fe 0。₁₅O和Ni₀₆₅Fe₀₃₅O)和氧化铁(Fe₂O₃)使用简单的化学浴沉积法合成,并通过扫描电子显微镜(SEM), x射线衍射(XRD),紫外可见光谱和x射线光电子能谱(XPS)进行系统表征。其中,Ni₀₈₅Fe₀。₁₅O表现出最好的OER性能,在10 mA cm⁻²时达到348 mV的低过电位,在替代海水(1 M KOH + 2.45 wt % NaCl)中运行24小时后,其塔菲斜率为52 mV dec⁻¹。这种优异的活性归因于其紧凑的纳米片形貌以及Ni 2 +和Fe³+之间的协同作用,经SEM和XPS证实,Ni 2 +和Fe +产生了晶格应变,增加了活性位点的密度。电化学表面积(ECSA)分析进一步揭示了在盐水条件下大量可达且稳定的活性位点,支持催化剂的固有活性。倪₀。₈₅Fe₀。₁₅O展示了与最先进的海水电解催化剂相媲美的OER性能和耐久性,强调了其可扩展和可持续制氢的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


