Spectroelectrochemical analysis of membrane permeation mechanisms of cell-penetrating peptide-modified fluorescent proteins
IF 4.5
2区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cell penetrating peptides (CPPs) can translocate substances into cells and have potential as molecular carriers in drug delivery system (DDS). In order to elucidate the cellular internalization mechanism of cargoes utilizing CPPs, the interfacial behavior of fluorescent protein, monomeric Azami-Green (mAG) modified with two types of CPPs, ε-poly-l-lysine (εPL) and octa-arginine (R8), was studied at the liquid|liquid interface as a model of biomembrane surfaces. CPP-unmodified mAG and R8-mAG showed the aqueous adsorption process at the water|1,2-dichloroethane interface. On the other hand, εPL-mAG was transferred across the interface accompanied by the adsorption steps at both sides of the interface. Spectroelectrochemical analysis indicated that the adsorption of mAG was facilitated at the phospholipid-modified biomimetic interface. In the presence of CPPs, the adsorption of mAG on the membrane surface was inhibited by competitive adsorption with εPL, whereas the phase transfer of mAG readily occurred due to the disturbance of the membrane structure by R8. Although the R8-mAG behaves in a similar membrane reaction mechanism to the unmodified mAG, the phase transfer efficiency of εPL-mAG was improved by suppressing the interaction with the phospholipid membrane. Therefore, the membrane permeation of mAG was successfully achieved by the modification with εPL. Present findings demonstrated that εPL is a promising CPP for the membrane permeation of proteins with a high hydrophilicity and molecular weight.
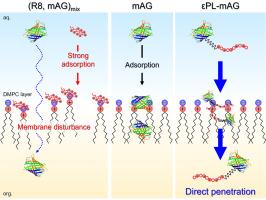
细胞穿透肽修饰荧光蛋白膜渗透机理的光谱电化学分析
细胞穿透肽(CPPs)具有转运物质进入细胞的功能,在药物传递系统(DDS)中具有作为分子载体的潜力。为了阐明利用CPPs的细胞内化机制,以两种CPPs (ε -聚赖氨酸(εPL)和八元精氨酸(R8))修饰的荧光蛋白单体Azami-Green (mAG)作为生物膜表面模型,研究了其在液体|液体界面上的界面行为。cpp -未改性mAG和R8-mAG在水- 1,2-二氯乙烷界面处表现出水吸附过程。另一方面,εPL-mAG在界面两侧的吸附过程中沿界面转移。光谱电化学分析表明,磷脂修饰的仿生界面有利于mAG的吸附。在CPPs存在的情况下,εPL的竞争性吸附抑制了mAG在膜表面的吸附,而R8对膜结构的干扰使mAG的相转移发生。虽然R8-mAG的膜反应机理与未修饰的mAG相似,但εPL-mAG通过抑制与磷脂膜的相互作用提高了相转移效率。因此,用εPL修饰mAG,成功地实现了mAG的膜透性。研究结果表明,εPL是一种具有高亲水性和高分子量蛋白质的膜渗透CPP。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioelectrochemistry
生物-电化学
CiteScore
9.10
自引率
6.00%
发文量
238
审稿时长
38 days
期刊介绍:
An International Journal Devoted to Electrochemical Aspects of Biology and Biological Aspects of Electrochemistry
Bioelectrochemistry is an international journal devoted to electrochemical principles in biology and biological aspects of electrochemistry. It publishes experimental and theoretical papers dealing with the electrochemical aspects of:
• Electrified interfaces (electric double layers, adsorption, electron transfer, protein electrochemistry, basic principles of biosensors, biosensor interfaces and bio-nanosensor design and construction.
• Electric and magnetic field effects (field-dependent processes, field interactions with molecules, intramolecular field effects, sensory systems for electric and magnetic fields, molecular and cellular mechanisms)
• Bioenergetics and signal transduction (energy conversion, photosynthetic and visual membranes)
• Biomembranes and model membranes (thermodynamics and mechanics, membrane transport, electroporation, fusion and insertion)
• Electrochemical applications in medicine and biotechnology (drug delivery and gene transfer to cells and tissues, iontophoresis, skin electroporation, injury and repair).
• Organization and use of arrays in-vitro and in-vivo, including as part of feedback control.
• Electrochemical interrogation of biofilms as generated by microorganisms and tissue reaction associated with medical implants.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


