A challenged spectral overlap resolution of oromucosal spray: A comparative study of PCR, PLS, and siPLS algorithms with sustainability assessment
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
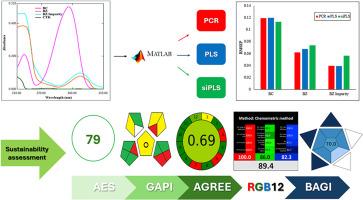
Three novel multivariate spectrophotometric methods namely Principal Component Regression (PCR), Partial Least Squares (PLS), and synergy intervals Partial Least Squares (siPLS) were developed for the successful resolution of spectrally overlapping signals of a quaternary mixture of benzocaine, benzydamine hydrochloride, cetalkonium chloride, and benzydamine hydrochloride official impurity (Impurity C). This work represents the first reported application of chemometric techniques to resolve this specific quaternary mixture in both its pure form and co-formulated dosage form.
The methods demonstrated good linearity and accuracy in analyzing the aforementioned compounds in laboratory-prepared mixtures and oromucosal spray. Linearity of the developed methods was found to be (3.00–15.00, 1.50–7.50 and 1.50–3.50 μg/mL) for benzocaine, benzydamine hydrochloride, and benzydamine hydrochloride impurity C (1-benzyl-1H-indazol-3-ol), correspondingly. The developed models were validated using internal and external data sets. Furthermore, statistical analysis did not reveal any substantial differences between our findings and those of the reported methods.
A comprehensive sustainability assessment, employing metrics such as Analytical Eco-Scale (AES), Green Analytical Procedure Index (GAPI), Analytical GREEnness metric (AGREE), Red-Green-Blue model (RGB12), and Blue Applicability Grade Index (BAGI), highlighted the advantages of the developed methods over conventional HPLC with respect to cost, time, and environmental impact. The developed methods achieved the following scores of 79, 0.69, 89.4, and 70 for AES, AGREE, RGB12, and BAGI, respectively.
These findings suggest that the proposed multivariate assisted spectrophotometric methods could offer a valuable alternative to HPLC for rapid and efficient quality control analysis of these compounds in pharmaceutical formulations.
口腔粘膜喷雾的光谱重叠分辨率受到挑战:PCR, PLS和siPLS算法与可持续性评估的比较研究
建立了三种新的多元分光光度法,即主成分回归(PCR)、偏最小二乘法(PLS)和协同区间偏最小二乘法(siPLS),用于成功分辨苯佐卡因、盐酸苄达明、氯化西塔克铵和盐酸苄达明官方杂质(杂质C)的四元混合物的光谱重叠信号。这项工作代表了首次报道的化学计量学技术的应用,以其纯形式和共配制剂型来解决这种特定的季铵盐混合物。该方法在实验室制备的混合物和口腔喷雾剂中具有良好的线性和准确性。苯佐卡因、盐酸苄达明和盐酸苄达明杂质C(1-苄基- 1h -吲哚唑-3-醇)的线性关系分别为(3.00 ~ 15.00、1.50 ~ 7.50和1.50 ~ 3.50 μg/mL。开发的模型使用内部和外部数据集进行验证。此外,统计分析并没有显示我们的发现与报道的方法之间有任何实质性的差异。采用分析生态尺度(AES)、绿色分析程序指数(GAPI)、分析绿色度指标(AGREE)、红绿蓝模型(RGB12)和蓝色适用性等级指数(BAGI)等指标进行的综合可持续性评估强调了所开发方法在成本、时间和环境影响方面优于传统HPLC的优势。所开发的方法在AES、AGREE、RGB12和BAGI方面的得分分别为79、0.69、89.4和70分。本研究结果表明,多变量辅助分光光度法可作为高效液相色谱法(HPLC)的替代方法,用于快速、高效地分析药物制剂中这些化合物的质量控制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


