Bergenin suppresses the changes of gut microbiota and colitis induced by dextran sulfate sodium in KM mice
IF 4
2区 农林科学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Objective
To investigate bergenin's (BE) modulatory effects on gut microbiota dysbiosis and its therapeutic mechanisms in dextran sulfate sodium (DSS)-induced colitis.
Methods
Kunming mice (n = 18) were randomized into Control, DSS (2.5 % DSS, 7 days), and DSS + BE (50 mg/kg/day) groups. Disease activity index (DAI), histopathology, and 16S rRNA sequencing of cecal microbiota were analyzed. Inflammatory markers (TLR4, α-SMA, iNOS) and epithelial proliferation (BrdU) were assessed.
Results
BE significantly attenuated colitis severity versus DSS group: reduced weight loss (23.1 % vs. 41.5 %,P < 0.05), lowered DAI scores (8.2 vs. 14.7,P < 0.01), and improved histopathology (2.1 vs. 4.8,P < 0.01). BE restored microbial α-diversity (Shannon index: 3.2 ± 0.4 vs. 2.1 ± 0.6,P < 0.01) and specifically increased anti-inflammatory Barnesiella abundance (4.2 % → 11.7 %,P < 0.01). Beta-diversity analysis revealed BE maintained microbiota structural stability (DSS + BE vs. Control dissimilarity <16 % vs. DSS >88 % at genus level). BE suppressed DSS-induced TLR4 overexpression and restored colonic epithelial proliferation (BrdU).
Conclusion
Bergenin ameliorates DSS-colitis by restoring gut microbiota homeostasis (notably enriching Barnesiella), suppressing TLR4-mediated inflammation, and promoting epithelial repair, highlighting its potential as an IBD therapeutic.
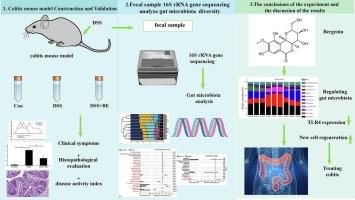
甜菜根素对葡聚糖硫酸钠诱导的KM小鼠肠道菌群变化及结肠炎有抑制作用
目的探讨甜菜根素(BE)对葡聚糖硫酸钠(DSS)所致结肠炎肠道菌群失调的调节作用及其治疗机制。方法将18只昆明小鼠随机分为对照组、DSS (2.5% DSS, 7 d)组和DSS + BE (50 mg/kg/d)组。分析盲肠菌群的疾病活动指数(DAI)、组织病理学和16S rRNA测序。检测炎症标志物(TLR4、α-SMA、iNOS)和上皮细胞增殖(BrdU)。结果与DSS组相比,be组结肠炎严重程度明显减轻:体重减轻(23.1%比41.5%,P <;0.05), DAI评分降低(8.2比14.7,P <;0.01),组织病理学改善(2.1 vs. 4.8,P <;0.01)。BE恢复微生物α-多样性(Shannon指数:3.2±0.4 vs. 2.1±0.6,P <;0.01)特异性增加抗炎巴氏菌丰度(4.2%→11.7%,P <;0.01)。β -多样性分析显示,BE保持了微生物群结构的稳定性(在属水平上,DSS + BE与对照的差异<; 16%与DSS >; 88%)。BE抑制dss诱导的TLR4过表达,恢复结肠上皮细胞增殖(BrdU)。结论甜菜根素可通过恢复肠道菌群稳态(尤其是富集巴氏菌)、抑制tlr4介导的炎症和促进上皮修复来改善dss -结肠炎,显示其治疗IBD的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Functional Foods
FOOD SCIENCE & TECHNOLOGY-
CiteScore
9.60
自引率
1.80%
发文量
428
审稿时长
76 days
期刊介绍:
Journal of Functional Foods continues with the same aims and scope, editorial team, submission system and rigorous peer review. We give authors the possibility to publish their top-quality papers in a well-established leading journal in the food and nutrition fields. The Journal will keep its rigorous criteria to screen high impact research addressing relevant scientific topics and performed by sound methodologies.
The Journal of Functional Foods aims to bring together the results of fundamental and applied research into healthy foods and biologically active food ingredients.
The Journal is centered in the specific area at the boundaries among food technology, nutrition and health welcoming papers having a good interdisciplinary approach. The Journal will cover the fields of plant bioactives; dietary fibre, probiotics; functional lipids; bioactive peptides; vitamins, minerals and botanicals and other dietary supplements. Nutritional and technological aspects related to the development of functional foods and beverages are of core interest to the journal. Experimental works dealing with food digestion, bioavailability of food bioactives and on the mechanisms by which foods and their components are able to modulate physiological parameters connected with disease prevention are of particular interest as well as those dealing with personalized nutrition and nutritional needs in pathological subjects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


