Green and high-efficiency sulfuric acid leaching strategy for enhanced recovery of rubidium and potassium from biotite
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The extraction of rubidium (Rb) and potassium (K) from biotite faces challenges due to the mineral's structural stability and high energy consumption in conventional methods. Herein, an environmentally friendly ion-exchange synergistic oxidation process was developed to enhance sulfuric acid leaching efficiency. By introducing sodium ions and magnesium ions as exchange agents and hydrogen peroxide as an oxidant, the layered structure of biotite was preserved while facilitating rubidium ion release. Key parameters, including sulfuric acid concentration, additive dosage, oxidant concentration, liquid-to-solid ratio, and temperature, were systematically optimized. Under optimal conditions (1.5 mol/L H2SO4, 0.4 g/g MgSO4, 0.2 g/g Na2SO4, 1 mol/L H2O2, L/S ratio 5 mL/g, 90 °C), a maximum Rb extraction efficiency of 95.2 % was achieved. By employing a 4-stage countercurrent leaching sequence followed by 3-stage countercurrent washing, the Rb+ concentration was ultimately increased to 1250 mg/L. Kinetic analysis revealed a two-stage mechanism: the initial 0–2 h phase was chemically controlled with an activation energy of 17.5 kJ/mol, while the subsequent 2–7 h phase transitioned to diffusion control with an activation energy of 16.5 kJ/mol. Multi-stage countercurrent leaching further concentrated rubidium ions to 1250 mg/L. DFT Calculations and structural analyses (XRD, FTIR, FIR, SEM-EDS, XPS) confirmed intact silicate frameworks and successful Na+/Mg2+ interlayer exchange, avoiding mineral decomposition. This strategy significantly reduces acid consumption, eliminates nitrate pollution, and demonstrates a promising and sustainable approach for the extraction of Rb and K from biotite.
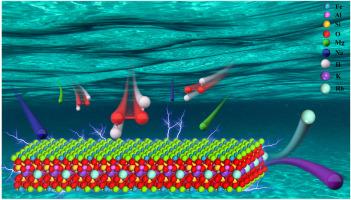
绿色高效硫酸浸出策略提高黑云母中铷和钾的回收率
从黑云母中提取铷(Rb)和钾(K)的传统方法由于其结构稳定性和高能耗而面临挑战。为了提高硫酸浸出效率,研究了一种环境友好型离子交换协同氧化工艺。通过引入钠离子和镁离子作为交换剂,过氧化氢作为氧化剂,保留了黑云母的层状结构,同时促进了铷离子的释放。对硫酸浓度、添加剂用量、氧化剂浓度、液固比、温度等关键参数进行了系统优化。在最佳条件(1.5 mol/L H2SO4、0.4 g/g MgSO4、0.2 g/g Na2SO4、1 mol/L H2O2、L/S比5 mL/g、90℃)下,Rb的提取率最高可达95.2%。采用4段逆流浸出,3段逆流洗涤,最终将Rb+浓度提高到1250 mg/L。动力学分析表明:初始0-2 h阶段为化学控制阶段,活化能为17.5 kJ/mol; 2-7 h阶段为扩散控制阶段,活化能为16.5 kJ/mol。多级逆流浸出进一步将铷离子浓缩至1250 mg/L。DFT计算和结构分析(XRD, FTIR, FIR, SEM-EDS, XPS)证实了完整的硅酸盐框架和成功的Na+/Mg2+层间交换,避免了矿物分解。该策略显著减少了酸消耗,消除了硝酸盐污染,为从黑云母中提取Rb和K展示了一种有前途的可持续方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


