DFT study on adsorption of Pb(Ⅱ) by biochar: focusing on molecular structure and alkali and alkaline earth metals
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
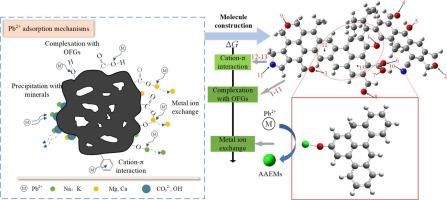
To investigate the Pb2+ adsorption characteristics of biochar, DFT (Density functional theory) calculation was employed as an effective method to evaluate the adsorption behavior. The three-dimensional structure of biochar was determined as C58H40N2O9 through 13C NMR, FTIR, and XPS analyses, which consisted of multiple triphenylene and biphenylene units linked by aliphatic carbons, functionalized with carbonyl, carboxyl, hydroxyl, and pyrrole groups on the surface. The adsorption sites were determined by molecular ESP analysis. DFT calculation showed that the adsorption performance was not only related to the type of functional groups, but also to the molecular structure in which the adsorption site was located. Configuration 3 showed the strongest Eads (−114 kJ mol−1) that Pb2+ achieved synergistic binding through simultaneous coordination to the carbonyl oxygen O(50) and adjacent aromatic carbons C(11)/C(12). The ion exchange capacity contributed by alkali and alkaline earth metals (AAEMs) presented on biochar followed K > Ca > Na > Mg. The ΔG of the ion exchange reaction was predominantly lower than that of Pb2+ adsorbed on biochar molecule through OFGs complexation and cation-π interaction, indicating the significant effect of AAEMs on the adsorption performance. Moreover, AAEMs coordinated on O atoms could enhance Pb-O bond strength during adsorption. These findings suggested that K/Ca enriched biochar was recommended for Pb2+ removal.

生物炭吸附Pb(Ⅱ)的DFT研究:以分子结构和碱土金属为重点
为了研究生物炭对Pb2+的吸附特性,采用密度泛函理论(DFT)计算方法对生物炭的吸附行为进行了评价。通过13C NMR、FTIR和XPS分析,确定了生物炭的三维结构为C58H40N2O9,由脂肪碳连接的多个三苯基和联苯单元组成,表面有羰基、羧基、羟基和吡咯基等官能团。通过分子电潜电分析确定了吸附位点。DFT计算表明,吸附性能不仅与官能团的类型有关,还与吸附位点所在的分子结构有关。构型3显示出最强的Eads(- 114 kJ mol - 1), Pb2+通过同时配位羰基氧O(50)和邻近的芳香碳C(11)/C(12)实现协同结合。碱金属和碱土金属(AAEMs)在生物炭上的离子交换能力;Ca 祝辞 Na 祝辞 毫克。离子交换反应的ΔG明显低于通过OFGs络合和阳离子-π相互作用吸附在生物炭分子上的Pb2+,表明AAEMs对吸附性能有显著影响。此外,在吸附过程中,与O原子配位的aaem可以增强Pb-O键的强度。这些结果表明,推荐富集K/Ca的生物炭去除Pb2+。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


