Precipitation as key parameter for early oral drug absorption predictions, Part II: Development of a predictive model
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
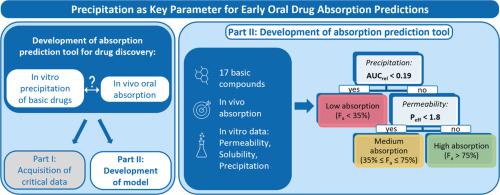
Accurate absorption predictions for new chemical entities (NCEs) are key in drug discovery, as they enable the early identification of compounds with suboptimal absorption characteristics. Common methodologies, such as the biopharmaceutical classification system (BCS), often lack quantitative insights, or they require extensive datasets at early stages, as seen in Physiologically Based Biopharmaceutics Modeling (PBBM). Furthermore, existing tools frequently overlook the impact of precipitation of weakly basic drugs on human absorption. To bridge this gap, we developed a novel oral absorption prediction tool that uses easily obtainable in vitro assay results while systematically investigating the relationship between in vitro precipitation and in vivo absorption. The underlying dataset, which is compiled in Part I of this study, includes in vivo human absorption data alongside in vitro permeability, solubility, and precipitation data for 17 basic compounds. In this part of the study, the dataset underwent a comprehensive statistical analysis. While regression analysis proved inadequate, a decision tree using the Classification and Regression Trees (CART) algorithm was constructed. The assessments from this algorithm led to the inclusion of both precipitation and permeability in the final decision tree, while solubility was excluded. This finding underscores the critical role and added value of in vitro precipitation compared to solubility. This intuitive tool is particularly well-suited for drug discovery, as it facilitates ranking and selection of drug candidates based on their oral absorption characteristics. Additionally, it can be easily adapted by other researchers, allowing for the incorporation of various in vitro assays and larger datasets to meet specific research needs. This flexibility paves the way for future advancements in the field.
沉淀作为早期口服药物吸收预测的关键参数,第二部分:预测模型的发展。
新化学实体(NCEs)的准确吸收预测是药物发现的关键,因为它们可以早期识别具有次优吸收特性的化合物。常见的方法,如生物制药分类系统(BCS),往往缺乏定量的见解,或者在早期阶段需要大量的数据集,如基于生理学的生物制药建模(PBBM)所见。此外,现有的工具经常忽略弱碱性药物沉淀对人体吸收的影响。为了弥补这一差距,我们开发了一种新的口服吸收预测工具,该工具使用易于获得的体外测定结果,同时系统地研究体外沉淀和体内吸收之间的关系。基础数据集汇编于本研究的第一部分,包括17种基本化合物的体内人体吸收数据以及体外渗透性、溶解度和沉淀数据。在这部分研究中,对数据集进行了全面的统计分析。考虑到回归分析的不足,利用分类回归树(CART)算法构建了一棵决策树。该算法的评估结果在最终决策树中包含了降水和渗透率,而排除了溶解度。这一发现强调了体外沉淀与溶解度相比的关键作用和附加价值。这种直观的工具特别适合于药物发现,因为它有助于根据药物的口服吸收特性对候选药物进行排名和选择。此外,它可以很容易地被其他研究人员调整,允许结合各种体外测定和更大的数据集,以满足特定的研究需求。这种灵活性为该领域未来的发展铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


