Development of an ophthalmic solution combining allogenic plasma with protective excipients for enhanced therapeutic role in the management of ocular surface diseases
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-07-20
DOI:10.1016/j.ejpb.2025.114813
引用次数: 0
Abstract
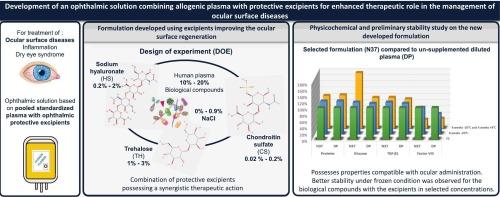
Ocular surface diseases (OSDs) are characterised by an instability of the tear film. In severe cases, these disorders have been improved by using human serum or plasma formulated into eye drops. These blood-derived-products present a biological composition similar to that of tears and contain beneficial factors that modulate inflammation and tissue regeneration. However, the supply of blood derivatives remains a challenge due to the potential microbiological risk, the quality and inter-individual heterogeneity of the biological samples used, and logistical difficulties. Thus, the idea behind this work was to use a standardised blood-derived product produced and checked industrially from a pool of donors, and to combine it with protective excipients known for their lubricating, anti-inflammatory, and regenerative properties offering new treatment opportunities for OSDs. Attention was also given to the non-bioaccumulative nature of the excipients. In order to select the concentrations of different compounds of interest in the ophthalmic solution, a design of experiment was used to establish a formulation with physicochemical properties (pH, osmolality, turbidity, viscosity) compatible with good ocular tolerance. Once defined, a physicochemical stability study testing pH, osmolality and dosage of biological factors, was carried out using the storage conditions usually used in clinical practice. A comparator without the additional compounds was also tested. The final formulation consisted of 20 % allogenic plasma, 0.5 % sodium hyaluronate (HS), 3 % trehalose (TH), 0.2 % chondroitin sulfate (CS) and 0.5 % sodium chloride (NaCl) (m/v). Its physicochemical properties should allow for good ocular tolerance. The data from the preliminary stability study favours storage at −20 °C for some of the biological factors measured.
一种结合同种异体血浆和保护性赋形剂的眼科溶液的开发,以增强眼表疾病的治疗作用
眼表疾病(OSDs)的特征是泪膜不稳定。在严重的情况下,通过使用配制成滴眼液的人血清或血浆,这些疾病得到改善。这些血液衍生产品具有类似于眼泪的生物成分,并含有调节炎症和组织再生的有益因子。然而,由于潜在的微生物风险、所使用生物样本的质量和个体间异质性以及后勤困难,血液衍生物的供应仍然是一个挑战。因此,这项工作背后的想法是使用一种标准化的血液来源产品,从一群献血者中生产和工业检查,并将其与具有润滑、抗炎和再生特性的保护性辅料结合起来,为osd提供新的治疗机会。还注意到赋形剂的非生物蓄积性。为了选择眼液中不同感兴趣的化合物的浓度,采用实验设计建立了一个物理化学性质(pH、渗透压、浊度、粘度)与良好的眼耐受性相容的配方。一旦定义,物理化学稳定性研究测试pH,渗透压和生物因子的剂量,在临床实践中通常使用的储存条件下进行。还测试了不含额外化合物的比较物。最终配方为20%同种异体血浆、0.5%透明质酸钠(HS)、3%海藻糖(TH)、0.2%硫酸软骨素(CS)和0.5%氯化钠(NaCl) (m/v)。它的物理化学性质应该允许良好的眼耐受性。从初步稳定性研究的数据来看,一些生物因素的测量结果更倾向于在- 20°C下储存。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


