Time-dependent evolution of morphologies and lattice defects in hydrothermal synthesized ceria-zirconia solid solutions for catalytic combustion of diesel soot
IF 7.7
2区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
In this work, the physical-chemical and morphological evolution of CeO2-ZrO2 solid solutions caused by hydrothermal processing time, and its effects on catalytic diesel soot elimination were studied. SEM and TEM results show that the CeO2-ZrO2 nanocrystal particles gradually aggregate with the extension of hydrothermal processing time. A moderate hydrothermal processing time can facilitate the engineering of catalyst morphology at micronmeter scale, however, excessive hydrothermal synthesis time would contrarily destroy the micrometer scaled catalyst morphological structure. The result of XPS, O2-TPD and H2-TPR analysis indicates that the hydrothermal synthesis process is conducive to generating lattice defects (oxygen vacancies) of CeO2-ZrO2 solid solution. This is a key factor for enhancing the concentration of surface-active oxygen species. The CeO2-ZrO2 catalyst prepared by 48 h of hydrothermal processing time (CZ-48 h) exhibits the highest concentration of surface-active oxygen species and oxygen vacancies. Interestingly, the CeO2-ZrO2 catalyst synthesized by 6 h of hydrothermal processing time (CZ-6 h) exhibits oxygen vacancy and surface-active oxygen levels that are close to the CZ-48 h, which is obviously more than the traditional co-precipitation CeO2-ZrO2 catalyst (CZ-0 h). The soot catalytic elimination performance of the CZ-6 h is also significantly superior to that of the CZ-0 h catalyst. Therefore, this study suggests that the short-time hydrothermal processing is an effective and low-cost strategy to create more oxygen vacancies and improve catalytic performance of CeO2-ZrO2 solid solution.
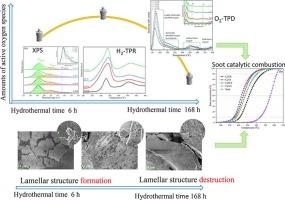
水热合成氧化铈-氧化锆固溶体催化燃烧中形貌和晶格缺陷随时间的演化
本文研究了水热处理时间对CeO2-ZrO2固溶体的物理化学和形态演化的影响,以及对催化柴油除烟的影响。SEM和TEM结果表明,随着水热处理时间的延长,CeO2-ZrO2纳米晶颗粒逐渐聚集。适度的水热处理时间有利于微米尺度催化剂形态的工程化,而过多的水热合成时间则会破坏微米尺度催化剂的形态结构。XPS、O2-TPD和H2-TPR分析结果表明,水热合成过程有利于生成CeO2-ZrO2固溶体的晶格缺陷(氧空位)。这是提高表面活性氧浓度的关键因素。水热处理时间为48 h (CZ-48 h)的CeO2-ZrO2催化剂表现出最高的表面活性氧浓度和氧空位。有趣的是,经过6 h水热处理时间(CZ-6 h)合成的CeO2-ZrO2催化剂的氧空位和表面活性氧水平接近CZ-48 h,明显高于传统共沉淀法的CeO2-ZrO2催化剂(CZ-0 h)。CZ-6 h的烟尘催化消除性能也明显优于CZ-0 h催化剂。因此,本研究表明,短时间水热处理是创造更多氧空位和提高CeO2-ZrO2固溶体催化性能的有效且低成本的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel Processing Technology
工程技术-工程:化工
CiteScore
13.20
自引率
9.30%
发文量
398
审稿时长
26 days
期刊介绍:
Fuel Processing Technology (FPT) deals with the scientific and technological aspects of converting fossil and renewable resources to clean fuels, value-added chemicals, fuel-related advanced carbon materials and by-products. In addition to the traditional non-nuclear fossil fuels, biomass and wastes, papers on the integration of renewables such as solar and wind energy and energy storage into the fuel processing processes, as well as papers on the production and conversion of non-carbon-containing fuels such as hydrogen and ammonia, are also welcome. While chemical conversion is emphasized, papers on advanced physical conversion processes are also considered for publication in FPT. Papers on the fundamental aspects of fuel structure and properties will also be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


