Thiobenzoic acid–catalyzed radical–radical cross–coupling between benzyl alcohols and benzophenones via hydrogen atom transfer
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The radical-radical cross-coupling of benzyl alcohols with benzophenones was successfully demonstrated by using thiobenzoic acid as a multifunctional photocatalyst. The reaction proceeded smoothly under visible-light irradiation to afford the corresponding diols in good yield and various functional groups were well tolerated. Furthermore, the catalytic system was also applicable to prenyl alcohol and benzylamine. Mechanistically, the excited state of thiobenzoate acts as a single electron reducing agent for benzophenones and the resulting sulfur radical promotes hydrogen atom abstraction from benzyl alcohols.
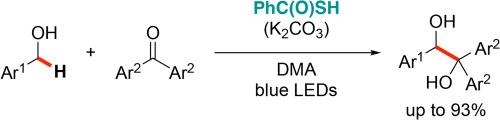
巯基苯甲酸通过氢原子转移催化苯甲醇和二苯甲酮之间的自由基-自由基交叉偶联
以硫代苯甲酸为多功能光催化剂,成功地证明了苯甲醇与二苯甲酮的自由基-自由基交叉偶联反应。该反应在可见光照射下进行顺利,得到相应的二醇,收率高,各种官能团耐受性好。此外,该催化体系也适用于戊醇和苄胺。从机理上讲,噻吩酸盐的激发态作为二苯甲酮的单电子还原剂,产生的硫自由基促进了苯甲醇中氢原子的提取。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


