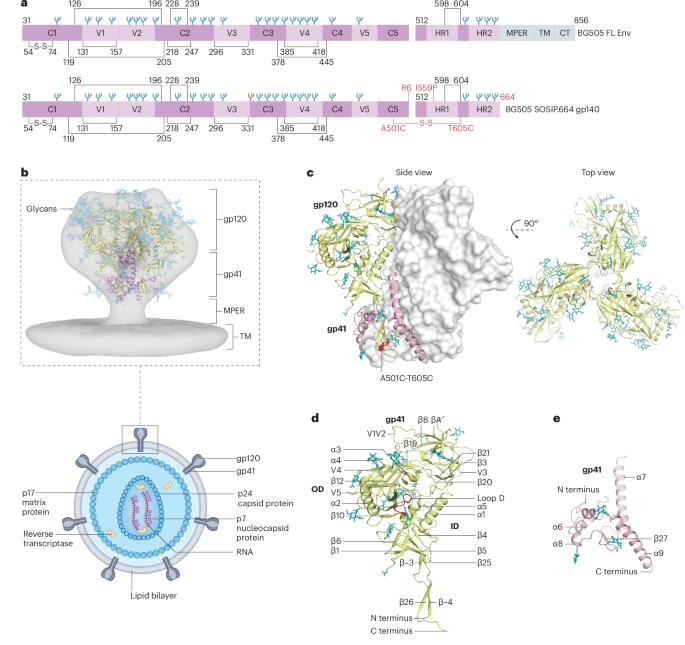
The HIV-1 envelope glycoprotein: structure, function and interactions with neutralizing antibodies
IF 103.3
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
To end the AIDS pandemic, an effective vaccine is sought to prevent new infections by inducing broadly active HIV-1 neutralizing antibodies. Monoclonal neutralizing antibodies can be administered therapeutically to people living with HIV-1 and preventively to those who are uninfected and at risk. Neutralizing antibodies block viral entry into susceptible cells by targeting the HIV-1 envelope glycoprotein, which mediates entry by membrane fusion. The envelope glycoprotein evades neutralizing antibody responses by multiple means, including extreme sequence variation and a dense protective glycan shield. Despite these impediments, many broadly active neutralizing human antibodies have been isolated, typically after years of HIV-1 infection. In this Review, we describe how such antibodies target distinct epitope clusters that cumulatively now cover most of the external surface of the envelope glycoprotein. These antibodies vary in potency, in the degree to which they reduce infectivity, in mechanism of action, and in structural basis, affinity and kinetics of binding. Broadly neutralizing antibody responses have, however, so far not been elicited by immunization with envelope glycoproteins. That situation may change though with the rapid advancement of structure-guided immunogen design strategies that engage germline versions of human antibodies and guide their maturation towards greater neutralization potency and breadth. In this Review, Klasse et al. explore the biogenesis and structure of the HIV-1 envelope glycoprotein (Env) and examine its functional role in viral entry. They also discuss how neutralizing antibodies interact with Env, the evolution of broadly neutralizing antibodies (bnAbs) and strategies to elicit bnAbs through germline-targeting immunogen design.

HIV-1包膜糖蛋白:结构、功能和与中和抗体的相互作用
为了结束艾滋病的流行,正在寻求一种有效的疫苗,通过诱导广泛活跃的艾滋病毒-1中和抗体来预防新的感染。单克隆中和抗体可用于治疗HIV-1感染者,也可用于预防未感染者和高危人群。中和抗体通过靶向HIV-1包膜糖蛋白阻断病毒进入易感细胞,而HIV-1包膜糖蛋白通过膜融合介导病毒进入。包膜糖蛋白通过多种方式逃避中和抗体反应,包括极端序列变异和密集的保护性聚糖屏蔽。尽管存在这些障碍,许多广泛活跃的中和性人类抗体已经被分离出来,通常是在HIV-1感染多年之后。在这篇综述中,我们描述了这些抗体如何靶向不同的表位簇,这些表位簇现在累积覆盖了包膜糖蛋白的大部分外表面。这些抗体在效力、降低传染性的程度、作用机制、结构基础、亲和力和结合动力学方面各不相同。然而,到目前为止,包膜糖蛋白免疫还没有引起广泛的中和抗体反应。然而,随着结构导向免疫原设计策略的快速发展,这种情况可能会发生变化,这些策略涉及人类抗体的种系版本,并引导它们走向更大的中和效力和广度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Reviews Microbiology
生物-微生物学
CiteScore
74.00
自引率
0.50%
发文量
149
审稿时长
6-12 weeks
期刊介绍:
At Nature Reviews Microbiology, our goal is to become the leading source of reviews and commentaries for the scientific community we cater to. We are dedicated to publishing articles that are not only authoritative but also easily accessible, supplementing them with clear and concise figures, tables, and other visual aids. Our objective is to offer an unparalleled service to authors, referees, and readers, and we continuously strive to maximize the usefulness and impact of each article we publish. With a focus on Reviews, Perspectives, and Comments spanning the entire field of microbiology, our wide scope ensures that the work we feature reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


