Powder-attached microneedles for the stable and effective transdermal delivery of clinically validated mRNA-LNP vaccine
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-07-19
DOI:10.1016/j.ejpb.2025.114811
引用次数: 0
Abstract
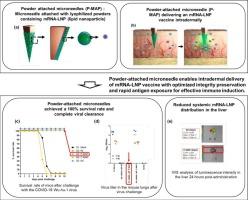
Microneedle-based delivery offers a promising strategy for administering mRNA-lipid nanoparticle (mRNA-LNP) vaccines via the skin; however, maintaining mRNA-LNP integrity and achieving effective immune responses remain challenging. This study presents a clinically relevant microneedle platform for the transdermal delivery of clinically validated mRNA-LNP vaccines using powder-attached microneedles (P-MAP) and coated microneedles (C-MAP). The mRNA-LNP vaccine, based on the formulation of Moderna’s clinically approved product, was prepared without alteration in their composition or manufacturing method. Lyophilization with stabilizers enabled the production of structurally preserved mRNA-LNP powders, which were physically attached onto the adhesive silicone surface of solid microneedles.
To evaluate delivery efficiency and quality, three key parameters were assessed: delivery efficiency (D), mRNA-LNP integrity (I), and release kinetics (R). Integrity (I) showed a strong correlation with humoral immune responses and in vivo protective efficacy. P-MAP exhibited a high I value along with rapid mRNA-LNP release, leading to efficient immune activation, complete protection, and efficient viral clearance in mice, whereas C-MAP, despite achieving comparable delivery efficiency, showed reduced structural integrity and slow-release kinetics, resulting in diminished immune responses and no protective effect. The lyophilized powder formulation also retained mRNA-LNP potency for over six months at 4 °C, demonstrating superior storage stability compared to conventional liquid formulations. Furthermore, this system significantly reduced off-target biodistribution to the liver, indicating a favorable safety profile.
These findings emphasize the need to optimize formulation stability and release kinetics for microneedle-based mRNA vaccine platforms. P-MAP provides a stable and clinically relevant strategy for safe and effective delivery of mRNA-LNP vaccine.
用于稳定有效经临床验证的mRNA-LNP疫苗透皮递送的粉末附着微针
基于微针的递送为通过皮肤给药mrna -脂质纳米颗粒(mRNA-LNP)疫苗提供了一种很有前途的策略;然而,维持mRNA-LNP的完整性和实现有效的免疫反应仍然具有挑战性。本研究提出了一种临床相关的微针平台,用于使用粉末附着微针(P-MAP)和包被微针(C-MAP)经皮递送经临床验证的mRNA-LNP疫苗。mRNA-LNP疫苗是在Moderna临床批准产品的基础上制备的,其成分或制造方法没有改变。使用稳定剂进行冻干,可以生产结构上保存完好的mRNA-LNP粉末,这些粉末被物理地附着在固体微针的粘性硅酮表面。为了评估递送效率和质量,评估了三个关键参数:递送效率(D), mRNA-LNP完整性(I)和释放动力学(R)。完整性(I)与体液免疫反应和体内保护功效有很强的相关性。P-MAP具有较高的I值,并能快速释放mRNA-LNP,从而在小鼠体内实现高效的免疫激活、完全的保护和高效的病毒清除,而C-MAP尽管具有相当的递送效率,但其结构完整性降低,释放动力学缓慢,导致免疫反应减弱,无保护作用。冻干粉制剂在4°C下也能保持mRNA-LNP效力超过6个月,与传统液体制剂相比,表现出优越的储存稳定性。此外,该系统显著减少了肝脏的脱靶生物分布,表明其具有良好的安全性。这些发现强调了优化基于微针的mRNA疫苗平台的配方稳定性和释放动力学的必要性。P-MAP为安全有效地递送mRNA-LNP疫苗提供了稳定和临床相关的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


