SECM stack imaging and full 3D modelling of asymmetric clusters of live cells
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Scanning Electrochemical Microscopy (SECM) has shown great strength as a bioanalytical technique for the characterization of single live cell topography, membrane permeability and extracellular reactive oxygen species. However, care must be taken to avoid the presence of adjacent cells in close proximity. Herein, we describe how these clusters of two or more cells may contribute to a combined signal. SECM is commonly coupled with simulated theoretical probe approach curves, allowing surface geometry or electrochemical reactivity to be quantified. Our novel experimental and simulation methodologies including tailored 3D SECM imaging of the live cells are reported here. These 3D modelling techniques allow the generation of 3D x-y-z cell profiles, cell surface maps at an electrode-cell separation, depth scan maps, probe approach curves to any cell spots of interest, and surface topography. The experimental quantification of cell height and topography was performed on cell clusters with an impermeable hydrophilic redox agent, ferrocenecarboxylate, for the deconvolution of these adjacent cell signals. As a proof of concept, experimental and theoretical results were compared to established model outputs. The characterization limits of commonly employed electrode sizes were assessed. Higher complexity cell geometries were explored for the first time, leading to the characterization of these cell clusters with any electrode size. The above developments are versatile, and further demonstrate the strength of SECM as a bioanalytical technique for monitoring cellular homeostasis.
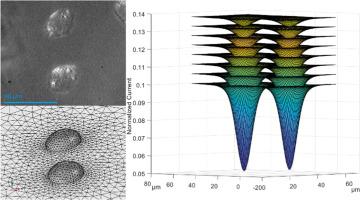
非对称活细胞簇的SECM堆栈成像和全三维建模
扫描电化学显微镜(SECM)作为一种生物分析技术,在单个活细胞的形貌、膜透性和细胞外活性氧的表征方面显示出巨大的优势。但是,必须注意避免相邻细胞的存在。在这里,我们描述了这些两个或更多细胞的集群如何有助于组合信号。SECM通常与模拟的理论探针接近曲线相结合,从而可以量化表面几何形状或电化学反应性。我们的新实验和模拟方法,包括量身定制的三维SECM活细胞成像报告在这里。这些3D建模技术允许生成3D x-y-z细胞轮廓、电极-细胞分离时的细胞表面图、深度扫描图、任何感兴趣的细胞点的探针接近曲线和表面形貌。细胞高度和地形的实验量化是用不渗透的亲水性氧化还原剂二茂铁羧酸盐在细胞簇上进行的,用于这些相邻细胞信号的反卷积。作为概念验证,实验和理论结果与建立的模型输出进行了比较。评估了常用电极尺寸的表征极限。更高复杂的细胞几何形状首次被探索,导致表征这些细胞团与任何电极尺寸。上述发展是多方面的,并进一步证明了SECM作为监测细胞稳态的生物分析技术的力量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


