Further exploring the tolerant region II: Identification of 2,4,5-trisubstituted pyrimidines as HIV-1 reverse transcriptase allosteric inhibitors with desirable antiviral activities and reduced cytotoxicity
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Here, we report a series of 2,4,5-trisubstituted pyrimidines as potent HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) by further exploring the potential tolerant region II within NNIBP. Most compounds were identified with potent inhibitory activity against HIV-1 wild-type (WT) strain with lower cytotoxicity. Among them, 14l exhibited the most outstanding antiviral activity (EC50 = 6.50–52.9 nM) against WT and a panel of mutant HIV-1 strains with much-reduced cytotoxicity (CC50 = 228 μM) and higher selectivity index (SI = 31434) than that of the lead 36a (CC50 = 45.6 μM; SI = 20550). Furthermore, the detailed molecular modeling studies revealed that the 3-thienyl substituent of 14l engages in multiple stable contacts with conserved residues within tolerant region II, providing a structural basis for the observed potent antiviral efficacy and enhanced anti-resistance profile. These findings collectively position 14l as a promising candidate for subsequent development as a novel lead compound.

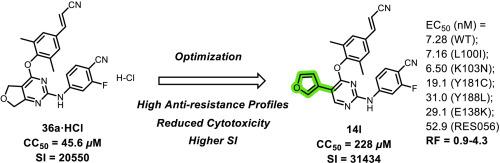
进一步探索耐受区II:鉴定2,4,5-三取代嘧啶作为HIV-1逆转录酶变构抑制剂具有理想的抗病毒活性和降低细胞毒性
在这里,我们通过进一步探索NNIBP中潜在的耐受区II,报道了一系列2,4,5-三取代嘧啶作为有效的HIV-1非核苷逆转录酶抑制剂(NNRTIs)。大多数化合物被鉴定为对HIV-1野生型(WT)菌株具有较低的细胞毒性的有效抑制活性。其中,14l对WT和一组HIV-1突变株表现出最显著的抗病毒活性(EC50 = 6.50 ~ 52.9 nM),其细胞毒性(CC50 = 228 μM)显著降低,选择性指数(SI = 31434)高于36a (CC50 = 45.6 μM);Si = 20550)。此外,详细的分子模拟研究表明,14l的3-噻吩基取代基与耐受区II内的保守残基进行了多次稳定的接触,为观察到的有效抗病毒效果和增强的抗抗性谱提供了结构基础。这些发现共同将14l定位为一种有希望的新型先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


