Peptide drug delivery: Permeation behaviour and intracellular fate of hydrophobic ion pairs in self-emulsifying drug delivery systems
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
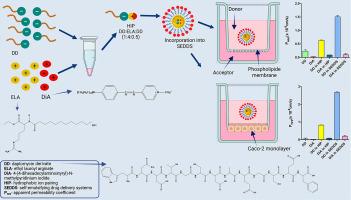
This study aimed to investigate the permeation behaviour and intracellular fate of hydrophobic ion pairs (HIP).
HIP were formed by combining a daptomycin-derived model peptide (DD) with ethyl lauroyl arginate (ELA) and lipophilic fluorescent dye 4-(4-dihexadecylaminostyryl)-N-methylpyridinium iodide (DiA). A representative HIP (DD: ELA: DiA, molar ratio 1:4:0.5) was incorporated into self-emulsifying drug delivery systems (SEDDS) and characterized for size, zeta potential, stability, hemolytic activity, cytotoxicity, and cellular uptake. Permeability was assessed using the Parallel Artificial Membrane Permeability Assay (PAMPA) model and Caco-2 monolayers.
SEDDS exhibited droplet sizes below 200 nm, a polydispersity index (PDI) < 0.4, positive surface charges, and high stability. Hemolysis studies indicated potential for endosomal escape, while dose-dependent toxicity became apparent after 4 and 24 h of incubation. Flow cytometry revealed enhanced cellular uptake: HIP and SEDDS increased internalization of DD by 12- and 32-fold, compared to free peptide. Permeation studies demonstrated marked improvements in DD transport. In the PAMPA assay, HIP and SEDDS increased passive diffusion by 2.8- and 6.5-fold. Similarly, in the Caco-2 model, HIP and SEDDS enhanced permeation by 17- and 57-fold, compared to free DD. DiA permeation remained minimal, suggesting that HIP disassociates intracellularly, allowing selective release of the peptide.
These findings confirm that HIP enhances membrane permeation of DD and dissociates after uptake. The combination of HIP and SEDDS presents a robust strategy for improving the oral bioavailability of peptide therapeutics.
多肽药物递送:自乳化药物递送系统中疏水离子对的渗透行为和细胞内命运。
本研究旨在探讨疏水离子对(HIP)的渗透行为和细胞内命运。由达托霉素衍生的模型肽(DD)与月桂酰精氨酸乙酯(ELA)和亲脂性荧光染料4-(4-二十六进胺苯乙烯基)- n-甲基碘化吡啶(DiA)结合形成HIP。将具有代表性的HIP (DD: ELA: DiA,摩尔比1:4:5 .5)加入到自乳化给药系统(SEDDS)中,并对其大小、ζ电位、稳定性、溶血活性、细胞毒性和细胞摄取进行了表征。采用平行人工膜通透性测定(PAMPA)模型和Caco-2单层膜评估通透性。SEDDS的液滴尺寸小于200 nm,多分散性指数(PDI) < 0.4,表面带正电荷,稳定性好。溶血研究表明可能有内体逃逸,而剂量依赖性毒性在4小时和24小时后变得明显。流式细胞术显示细胞摄取增强:与游离肽相比,HIP和SEDDS使DD的内在化增加了12倍和32倍。渗透研究表明,DD转运有显著改善。在PAMPA试验中,HIP和SEDDS使被动扩散增加2.8倍和6.5倍。同样,在Caco-2模型中,与游离DD相比,HIP和SEDDS的通透性分别提高了17倍和57倍。DiA的通透性仍然很低,这表明HIP在细胞内解离,允许肽的选择性释放。这些发现证实,HIP增强了DD的膜透性,并在摄取后解离。HIP和SEDDS的结合为改善肽治疗药物的口服生物利用度提供了一个强有力的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


