From surface design to cellular Response: Insights into aryl-functionalized iron oxide nanoparticles with and without protein corona
Q3 Materials Science
引用次数: 0
Abstract
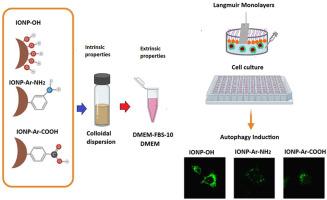
Iron oxide magnetic nanoparticles (IONPs) are widely utilized in biomedical and industrial applications due to their unique properties, including biocompatibility, superparamagnetism, and ease of functionalization. However, their behavior in biological environments is heavily influenced by surface functionalization and the formation of the protein corona. This study investigates the impact of aryl-functionalization of iron oxide nanoparticles with carboxylic and amine groups on colloidal stability, protein corona formation, and biological interactions. The IONPs were synthesized and characterized for their physicochemical properties, including size, zeta potential, magnetic properties, and dispersibility in different media. The interaction of the nanoparticles with dipalmitoylphosphatidylcholine monolayers, as a model membrane, was evaluated. Cytotoxicity and autophagy induction were assessed in Chinese hamster ovary (CHO-K1) and cervical cancer (HeLa) cells, respectively. The results demonstrate that surface functionalization significantly alters protein corona composition, which in turn modulates nanoparticle stability, cellular uptake, and biological responses. The aryl-functionalized nanoparticles exhibited reduced interactions with cell membranes compared to unfunctionalized counterparts, and also lower autophagy induction, emphasizing the importance of surface design in minimizing adverse effects.
从表面设计到细胞反应:有或没有蛋白冠的芳基功能化氧化铁纳米颗粒的见解
氧化铁磁性纳米颗粒(IONPs)由于其独特的特性,包括生物相容性、超顺磁性和易于功能化,在生物医学和工业应用中得到了广泛的应用。然而,它们在生物环境中的行为在很大程度上受到表面功能化和蛋白质冠形成的影响。本研究探讨了含羧基和胺基的氧化铁纳米颗粒的芳基功能化对胶体稳定性、蛋白质电晕形成和生物相互作用的影响。合成并表征了IONPs的物理化学性质,包括尺寸、zeta电位、磁性和在不同介质中的分散性。研究了纳米颗粒与双棕榈酰磷脂酰胆碱单层膜的相互作用。分别对中国仓鼠卵巢(CHO-K1)和宫颈癌(HeLa)细胞进行细胞毒性和自噬诱导。结果表明,表面功能化显著改变了蛋白质冠的组成,从而调节纳米颗粒的稳定性、细胞摄取和生物反应。与未功能化的纳米颗粒相比,芳基功能化的纳米颗粒与细胞膜的相互作用减少,自噬诱导也降低,这强调了表面设计对最小化不良反应的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JCIS open
Physical and Theoretical Chemistry, Colloid and Surface Chemistry, Surfaces, Coatings and Films
CiteScore
4.10
自引率
0.00%
发文量
0
审稿时长
36 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


