Exosomes derived from baicalin-pretreated bone marrow mesenchymal stem cells inactivate the TLR4/MyD88/NF-kB pathway to improve asthma
IF 2.3
4区 医学
Q3 IMMUNOLOGY
引用次数: 0
Abstract
Background
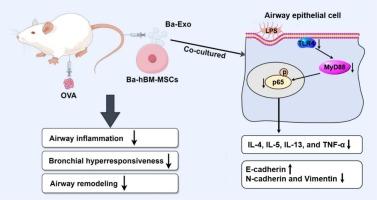
Baicalin, a natural compound isolated from the root of Scutellaria baicalensis Georgi, has been shown to have various pharmacological effects on lung diseases including asthma. Recently, research has suggested that baicalin combined with exosomes may have significant potential against disease development. The present work analyzes the effects of exosomes derived from baicalin-pretreated bone marrow mesenchymal stem cells (BMSCs) on asthma and the underlying mechanism.
Methods
BALB/c mice were sensitized with ovalbumin (OVA) through intraperitoneal injection to establish an animal model of asthma. Human bronchial epithelial cells (16HBE) were exposed to lipopolysaccharide to mimic a cell model of asthma. The pathological conditions of lung tissues in OVA-induced mice were analyzed by haematoxylin and eosin staining assays. Masson staining and quantification analysis were conducted to analyze percentage of collagen fibers in lung tissues of OVA-induced mice. The Wright-Giemsa assay was used to determine the number of eosinophils, neutrophils, lymphocytes and macrophages. Enzyme-linked immunosorbent assays were performed to analyze expression levels of inflammatory factors including IL-4, IL-5, IL-13 and TNF-α levels. The values of airway resistance (Rrs), elastance (Ers) and compliance (Crs) were recorded for analyzing airway hyperresponsiveness through the FlexiVent system. Protein expression was analyzed by immunohistochemistry (IHC) and/or western blotting assay.
Results
Ovalbumin (OVA) pretreatment increased airway inflammation, airway hyperresponsiveness, collagen deposition and epithelial-mesenchymal transition (EMT) in mice, however, these phenomena were significantly improved after treatment with baicalin-pretreated BMSC exosomes. Lipopolysaccharide (LPS)-induced 16HBE cells showed increased levels of interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-13 (IL-13) and tumor necrosis factor-α (TNF-α), elevated N-cadherin and Vimentin protein expression, and decreased E-cadherin protein expression, whereas these LPS-induced effects were relieved after treatment with baicalin-pretreated BMSC exosomes. Additionally, protein expression of toll-like receptor 4 (TLR4), myeloid differentiation primary response protein 88 (MyD88) and phosphor p65 (p-p65) was upregulated in lung tissues of OVA-induced mice and LPS-stimulated 16HBE cells, but these phenomena were counteracted following exosomes treatment from baicalin-pretreated BMSCs.
Conclusion
Exosomes derived from baicalin-pretreated BMSCs ameliorated airway inflammation, airway hyperresponsiveness and airway remodeling after asthma by inactivating the TLR4/MyD88/nuclear factor kappa B pathway, providing a therapeutic strategy for asthma.
来自黄芩苷预处理的骨髓间充质干细胞的外泌体灭活TLR4/MyD88/NF-kB通路以改善哮喘
黄芩苷是一种从黄芩根中分离得到的天然化合物,对包括哮喘在内的肺部疾病具有多种药理作用。最近的研究表明,黄芩苷与外泌体联合使用可能具有显著的抗疾病发展潜力。本研究分析了黄芩苷预处理骨髓间充质干细胞(BMSCs)外泌体对哮喘的影响及其机制。方法腹腔注射卵清蛋白致敏balb /c小鼠,建立哮喘动物模型。人支气管上皮细胞(16HBE)暴露于脂多糖以模拟哮喘细胞模型。采用苏木精和伊红染色法分析ova诱导小鼠肺组织的病理情况。采用Masson染色和定量分析ova诱导小鼠肺组织中胶原纤维的百分比。Wright-Giemsa法测定嗜酸性粒细胞、中性粒细胞、淋巴细胞和巨噬细胞的数量。采用酶联免疫吸附法分析炎症因子IL-4、IL-5、IL-13和TNF-α的表达水平。记录气道阻力(Rrs)、弹性(Ers)和顺应性(Crs)值,通过FlexiVent系统分析气道高反应性。通过免疫组织化学(IHC)和/或免疫印迹法分析蛋白表达。结果卵白蛋白(OVA)预处理可增加小鼠气道炎症、气道高反应性、胶原沉积和上皮间质转化(EMT),而黄芩苷预处理BMSC外泌体可显著改善上述现象。脂多糖(LPS)诱导的16HBE细胞白细胞介素-4 (IL-4)、白细胞介素-5 (IL-5)、白细胞介素-13 (IL-13)和肿瘤坏死因子-α (TNF-α)水平升高,N-cadherin和Vimentin蛋白表达升高,E-cadherin蛋白表达降低,而黄芩苷预处理的BMSC外泌体处理后,LPS诱导的这些作用得到缓解。此外,ova诱导小鼠和lps刺激的16HBE细胞肺组织中toll样受体4 (TLR4)、髓样分化初级反应蛋白88 (MyD88)和磷酸化蛋白p65 (p-p65)的蛋白表达上调,但这些现象在黄芩苷预处理的骨髓间充质干细胞外泌体处理后被抵消。结论黄芩苷预处理的骨髓间充质干细胞外泌体通过灭活TLR4/MyD88/核因子κ B通路,改善哮喘后气道炎症、气道高反应性和气道重塑,为哮喘的治疗提供了一种策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunobiology
医学-免疫学
CiteScore
5.00
自引率
3.60%
发文量
108
审稿时长
55 days
期刊介绍:
Immunobiology is a peer-reviewed journal that publishes highly innovative research approaches for a wide range of immunological subjects, including
• Innate Immunity,
• Adaptive Immunity,
• Complement Biology,
• Macrophage and Dendritic Cell Biology,
• Parasite Immunology,
• Tumour Immunology,
• Clinical Immunology,
• Immunogenetics,
• Immunotherapy and
• Immunopathology of infectious, allergic and autoimmune disease.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


