TMEM65 functions as the mitochondrial Na+/Ca2+ exchanger
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Mitochondria export Ca2+ via Na+/Ca2+ exchange machinery (mito-NCX) to regulate intracellular Ca2+ signalling and mitochondrial Ca2+ homeostasis. TMEM65 has recently been implicated as essential for mito-NCX, but its mechanisms and roles remain unclear. Here we show that TMEM65 depletion severely impairs mito-NCX. TMEM65 is highly expressed in the heart and brain but absent in the liver, correlating with mito-NCX activity in these tissues. Biochemical and functional analyses reveal that TMEM65 forms a homodimer, containing plausible ion-coordinating residues critical for function. Heterologous expression of TMEM65 induces Na+/Ca2+ exchange in cells lacking native mito-NCX activity. Moreover, purified, liposome-reconstituted TMEM65 exhibits key mito-NCX features. We further identify the binding site for CGP-37157, a potent, widely used mito-NCX inhibitor. Finally, TMEM65 deletion elevates mitochondrial Ca2+ and primes mitochondria to permeability transition. These findings firmly establish TMEM65 as the protein mediating mito-NCX, offering a new therapeutic target for diseases associated with mitochondrial Ca2+ dysregulation. Zhang, Chang et al. identify the protein TMEM65 as the mitochondrial Na+/Ca2+ exchanger, showing that it dimerizes to mediate ion transport and contains a binding site for a well-known inhibitor of mitochondrial Na+/Ca2+ exchange.
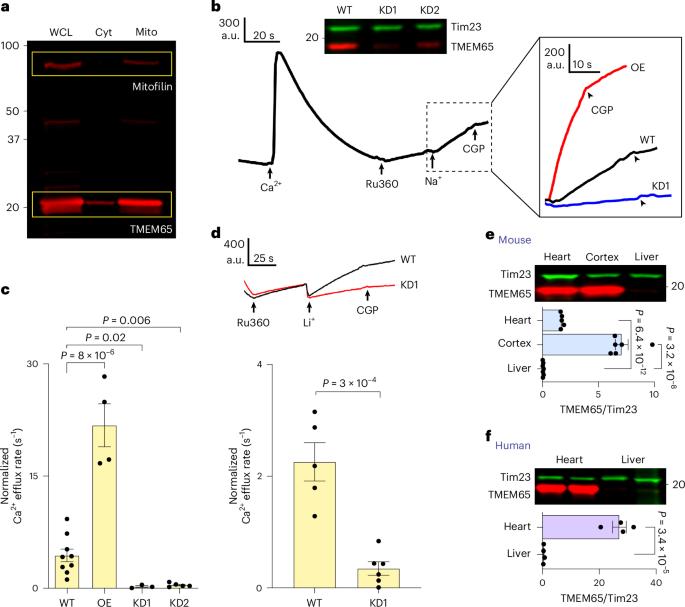
TMEM65作为线粒体Na+/Ca2+交换器
线粒体通过Na+/Ca2+交换机制(mito-NCX)输出Ca2+,调节细胞内Ca2+信号和线粒体Ca2+稳态。TMEM65最近被认为对mitto - ncx至关重要,但其机制和作用尚不清楚。我们发现TMEM65耗损严重损害mito-NCX。TMEM65在心脏和大脑中高表达,但在肝脏中不表达,这与这些组织中的mito-NCX活性相关。生化和功能分析表明,TMEM65形成一种同型二聚体,含有对功能至关重要的离子配位残基。TMEM65的异源表达在缺乏天然mito-NCX活性的细胞中诱导Na+/Ca2+交换。此外,纯化的脂质体重构的TMEM65表现出mitto - ncx的关键特征。我们进一步确定了CGP-37157的结合位点,CGP-37157是一种有效的,广泛使用的mito-NCX抑制剂。最后,TMEM65缺失会提高线粒体Ca2+并启动线粒体向通透性转变。这些发现坚定地确立了TMEM65是介导mito-NCX的蛋白,为线粒体Ca2+失调相关疾病提供了新的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


