Concerted changes in Epithelium and Stroma: a multi-scale, multi-omics analysis of progression from Barrett’s Esophagus to adenocarcinoma
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Esophageal adenocarcinoma arises from Barrett’s esophagus, a metaplastic condition. Multi-omics profiling, integrating single-cell transcriptomics, extracellular matrix proteomics, tissue mechanics and spatial proteomics of the paths of progression from squamous epithelium through metaplasia, dysplasia to adenocarcinoma, in 107 samples from 26 patients in two independent cohorts, defined shared and patient-specific progression characteristics. Metaplastic replacement of epithelial cell composition and architecture was paralleled by changes in stromal cells, extracellular matrix (ECM) and tissue stiffness. This change in pre-cancerous metaplasia was already accompanied by appearance of fibroblasts with the molecular characteristics of carcinoma-associated fibroblasts. These fibroblasts produced the immunosuppressive protein POSTN, whose expression shifted from vascular to stromal cells, consistent with the emergence of an immunosuppressive microenvironment evident in cell neighborhoods enriched for immunoregulatory NK and Treg cells. Thus, Barrett’s esophagus progresses as a coordinated multi-component system, supporting treatment paradigms that go beyond targeting cancerous cells to incorporate stromal reprogramming.
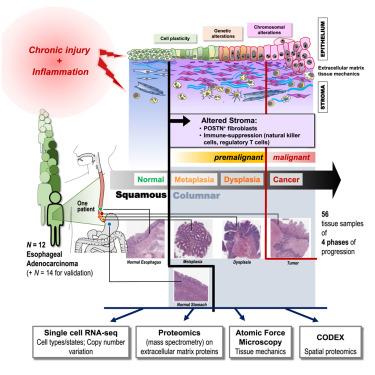
上皮和间质协同变化:Barrett食管向腺癌进展的多尺度、多组学分析
食管腺癌起源于Barrett食管,是一种化生疾病。多组学分析,整合单细胞转录组学、细胞外基质蛋白质组学、组织力学和空间蛋白质组学,从两个独立队列的26名患者的107个样本中,从鳞状上皮到化生、不典型增生到腺癌的进展路径,定义了共同的和患者特异性的进展特征。上皮细胞组成和结构的化生替代与基质细胞、细胞外基质(ECM)和组织刚度的变化是平行的。癌前化生的这种变化已经伴随着具有癌相关成纤维细胞分子特征的成纤维细胞的出现。这些成纤维细胞产生免疫抑制蛋白POSTN,其表达从血管细胞转移到基质细胞,这与免疫抑制微环境的出现一致,这种微环境明显存在于富含免疫调节NK细胞和Treg细胞的细胞社区中。因此,Barrett食道作为一个协调的多组分系统发展,支持超越靶向癌细胞的治疗范式,结合基质重编程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


