Neuroendocrine control of intestinal regeneration through the vascular niche in Drosophila
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
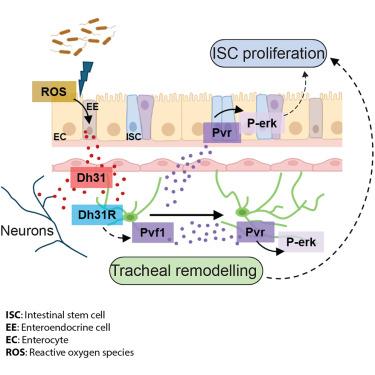
Robust and controlled intestinal regeneration involves reciprocal interactions between the intestinal epithelium and its microenvironment. Here, we identify signaling between enteroendocrine (EE) cells, vasculature-like trachea, and neurons, which drives regional and global stem cell proliferation during adult intestinal regeneration in Drosophila. Reactive oxygen species (ROS) from midgut cells promote production and secretion of diuretic hormone 31 (Dh31), from anterior midgut EE cells. EE and neuronal Dh31 activate tracheal Dh31 receptor, leading to the production of the vascular endothelial growth factor (VEGF)- and platelet-derived-growth-factor (PDGF)-like ligand Pvf1. Pvf1 induces tracheal remodeling and intestinal stem cell (ISC) proliferation through autocrine and paracrine Pvr/mitogen-activated protein kinase (MAPK) signaling, respectively. While EE Dh31 exerts broad control of ISC proliferation throughout the midgut, effects of the neuronal source of the ligand appear restricted to the posterior midgut. Collectively, our work discovered an EE/neuronal/vascular signaling network, controlling global and domain-specific ISC proliferation during adult intestinal regeneration.
果蝇血管生态位对肠道再生的神经内分泌调控
强健和可控的肠道再生涉及肠上皮及其微环境之间的相互作用。在这里,我们确定了肠内分泌(EE)细胞、血管样气管和神经元之间的信号传导,这些信号传导在果蝇成年肠道再生过程中驱动区域和全局干细胞增殖。来自中肠细胞的活性氧(ROS)促进来自前中肠EE细胞的利尿激素31 (Dh31)的产生和分泌。EE和神经元Dh31激活气管Dh31受体,导致血管内皮生长因子(VEGF)和血小板衍生生长因子(PDGF)样配体Pvf1的产生。Pvf1分别通过自分泌和旁分泌Pvr/丝裂原活化蛋白激酶(MAPK)信号诱导气管重塑和肠干细胞(ISC)增殖。虽然EE Dh31对整个中肠的ISC增殖具有广泛的控制作用,但该配体的神经元来源的影响似乎仅限于后中肠。总的来说,我们的工作发现了一个EE/神经元/血管信号网络,在成人肠道再生过程中控制全局和特定域的ISC增殖。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


