Phytochemical profiling of Bai traditional food: Eight previously undescribed meroterpenoids derivatives with cytotoxic potential from the flowers of Rhododendron pachypodum
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The flowers of Rhododendron pachypodum (Ericaceae), a traditional edible vegetable of the Bai ethnic group in Yunnan Province, China, are widely used both as food and medicine. A systematic phytochemical survey was conducted to fill the knowledge gap regarding its bioactive constituents. Fourteen meroterpenoids, including eight previously undescribed meroterpenoid compounds, rubiginosin I–J (1–2), (±)-rubiginosin K (3), (±)-rubiginosin L (4), (±)-rubiginosin N (5), and six known analogues (6–11), were structurally elucidated using a combination of spectroscopic techniques (1D/2D NMR, HRESIMS, UV, IR). The absolute configurations were unambiguously established by X-ray crystallography and ECD calculations. Selected compounds were assessed for their cytotoxicity, α-glucosidase inhibition, and DPPH radical scavenging activities. Compound 6 demonstrated cytotoxic effects against HepG2 and HeLa cell lines, with IC50 values of 40.56 μM and 57.43 μM, respectively. Compound 7 exhibited a slight cytotoxic activity against A549 cell lines, with an inhibition rate of 60 %, while compound 8 demonstrated mild activity against both A549 and MDA-MB-468 cells, with inhibition rates of 70 % and 65 %, respectively, at a concentration of 100 μM. In the α-glucosidase inhibition assay, compounds 2–11 showed weak activity at 100 μg/mL. Similarly, in the DPPH assay, these compounds exhibited poor free radical scavenging activity at 100 μg/mL. This study not only expands the chemical diversity of meroterpenoids in Rhododendron pachypodum, but also provides the first evidence of their cytotoxic potential, although structural optimization is required to enhance bioactivity.
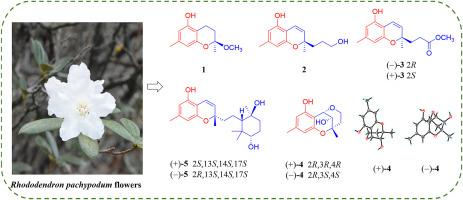
白族传统食品的植物化学分析:八种先前未被描述的具有细胞毒性的红杜鹃花衍生物。
厚杜鹃花(杜鹃花科)是中国云南省白族的一种传统食用蔬菜,被广泛用作食物和药物。进行了系统的植物化学调查,以填补有关其生物活性成分的知识空白。利用光谱技术(1D/2D NMR、HRESIMS、UV、IR)对14个巯基萜类化合物进行了结构鉴定,其中包括8个先前未被描述的巯基萜类化合物,即红比基苷I-J(1-2)、(±)-红比基苷K(3)、(±)-红比基苷L(4)、(±)-红比基苷N(5)和6个已知的类似物(6-11)。通过x射线晶体学和ECD计算,确定了绝对构型。对选定的化合物进行了细胞毒性、α-葡萄糖苷酶抑制和DPPH自由基清除活性的评估。化合物6对HepG2和HeLa细胞株表现出细胞毒作用,IC50值分别为40.56 μM和57.43 μM。化合物7对A549细胞有轻微的细胞毒活性,抑制率为60%,而化合物8对A549和MDA-MB-468细胞均有轻微的细胞毒活性,在100 μM浓度下,抑制率分别为70%和65%。在α-葡萄糖苷酶抑制实验中,化合物2-11在100 μg/mL时表现出弱活性。同样,在DPPH实验中,这些化合物在100 μg/mL时表现出较差的自由基清除活性。本研究不仅扩大了肿杜鹃(Rhododendron pachypodum)中meroterpenoids的化学多样性,而且首次提供了其细胞毒性潜力的证据,尽管结构优化需要提高生物活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


