Transport across membrane meets biophysics to unveil the mechanism of action of a novel gH625 analogue
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Cell-penetrating peptides are widely used in drug delivery for their ability to facilitate the transport of nanomaterials inside the cell. We previously studied the gH-625 for its ability to cross cell membranes, delivering various cargos into different cell types. In this study, since gH-625 suffers from low proteolytic stability, we identified the main cleavage sites after incubation with the enzyme chymotrypsin, and l-amino acids at these sites were replaced with their d-enantiomers, which share similar physicochemical properties but have distinct biological roles. Four peptides, namely gH-w10, gH-l7, gH-y13, and gH-combi, were designed and synthesized. Their biosafety profiles were evaluated in both normal and cancer cell lines and no significant toxic effects were revealed at the tested concentrations. Subsequently, we assessed their cell-penetrating ability by evaluating cellular uptake through fluorescence microscopy and investigated their mechanism of action in a model system of liposomes, measuring fusogenic activity, peptide insertion into the lipid bilayer, and leakage activity. The impact of the d-amino acid substitution on secondary structure was explored by circular dichroism and nuclear magnetic resonance studies. Finally, in vitro safety profiling data of the gH-625 and its most promising derivative gH-combi were further confirmed in vivo using a chicken embryo model.
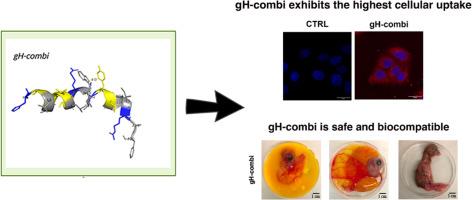
跨膜转运与生物物理学相结合,揭示了一种新型gH625类似物的作用机制。
细胞穿透肽因其促进纳米材料在细胞内运输的能力而广泛应用于药物递送。我们之前研究了gH-625跨越细胞膜的能力,将各种货物输送到不同的细胞类型。在本研究中,由于gH-625的蛋白水解稳定性较低,我们在与胰凝乳酶孵育后确定了主要的裂解位点,并将这些位点上的l -氨基酸替换为它们的d对映体,它们具有相似的物理化学性质,但具有不同的生物学作用。设计并合成了gH-w10、gh - 17、gH-y13和gH-combi四种多肽。在正常和癌细胞系中对它们的生物安全性进行了评估,在测试浓度下没有发现明显的毒性作用。随后,我们通过荧光显微镜评估细胞摄取来评估它们的细胞穿透能力,并研究了它们在脂质体模型系统中的作用机制,测量了促聚变活性、肽插入脂质双分子层和渗漏活性。通过圆二色性和核磁共振研究探讨了d -氨基酸取代对二级结构的影响。最后,利用鸡胚模型进一步验证了gH-625及其最有前途的衍生物gH-combi的体外安全性分析数据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


