Bioactive diamond scaffolds support neuronal survival and axonal growth
Q1 Medicine
引用次数: 0
Abstract
Injury to the central nervous system (CNS) can have devastating consequences for the individual, and strategies to promote endogenous axonal regeneration may be a promising future therapeutic avenue. In the case of spinal cord injury, one approach is to generate a scaffold-bridge across the injury site, through which the neuronal axons can grow and reconnect. Inspired by the various properties of diamond, including its chemical inertness, we proposed a strategy in which synthetic diamond scaffolds were coated with proteins with beneficial properties to promote biocompatibility of the scaffolds towards neurons. Here, we demonstrated that bare, non-coated diamond scaffolds, when terminated with either oxygen or hydrogen, were unable to adhere to the human embryonic stem cell-derived interneurons in culture. In contrast, oxygen terminated protein-coated scaffolds (i.e., bioactive diamond scaffold) efficiently enabled neuronal attachment and supported the survival, migration, and neurite elongation across an induced injury gap in culture. Further, hydrogen terminated bioactive scaffolds also promoted cell adhesion, migration, and neurite elongation upon injury, but not as efficiently as oxygen-terminated bioactive scaffolds. With this data we suggest that bioactive synthetic diamond scaffolds could provide a valuable tool for future therapeutic strategies in the context of CNS injuries.
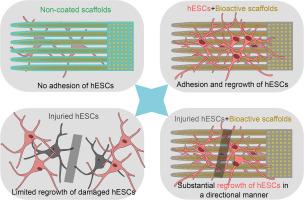
生物活性金刚石支架支持神经元存活和轴突生长
中枢神经系统(CNS)损伤对个体具有毁灭性的后果,促进内源性轴突再生的策略可能是未来有希望的治疗途径。在脊髓损伤的情况下,一种方法是在损伤部位产生一个支架桥,通过它神经元轴突可以生长和重新连接。受金刚石的各种特性(包括其化学惰性)的启发,我们提出了一种策略,在合成金刚石支架上涂覆具有有益特性的蛋白质,以促进支架对神经元的生物相容性。在这里,我们证明了裸的、无涂层的金刚石支架,当用氧或氢终止时,不能附着在培养的人类胚胎干细胞衍生的中间神经元上。相比之下,氧终止蛋白包被支架(即生物活性金刚石支架)有效地使神经元附着,并支持在培养中诱导损伤间隙中的存活、迁移和神经突伸长。此外,氢端生物活性支架在损伤后也能促进细胞粘附、迁移和神经突伸长,但效果不如氧端生物活性支架。根据这些数据,我们认为生物活性合成金刚石支架可以为未来中枢神经系统损伤的治疗策略提供有价值的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Engineered regeneration
Biomaterials, Medicine and Dentistry (General), Biotechnology, Biomedical Engineering
CiteScore
22.90
自引率
0.00%
发文量
0
审稿时长
33 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


