Elastic properties of force-transmitting linkages determine multistable mechanosensitive behaviour of cell adhesion
IF 18.4
1区 物理与天体物理
Q1 PHYSICS, MULTIDISCIPLINARY
引用次数: 0
Abstract
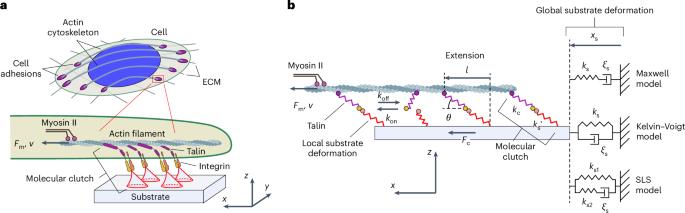
Cells can sense and respond to their environment. Central to this process is the formation of molecular clutches, which are dynamic linkages between the extracellular matrix and the actin cytoskeleton mediated by integrins and adaptor proteins. Although it is well known that force-dependent interactions between molecular-clutch components are essential for sensing substrate rigidity, the influence of nonlinear adaptor-protein elasticity is poorly understood. Here we show that adaptor-protein elasticity and local interactions between molecular clutches and the extracellular matrix are key to cellular perception of substrate stiffness. We present a semi-analytical theory that integrates experimentally measured force responses of adaptor proteins to describe cell-adhesion sensing. We propose that molecular clutches probe local mechanical substrate properties and collectively function as a differential that allows a retrograde actin flow and substrate movement to occur at different rates while maintaining a stable mechanical coupling between them. Our model reproduces experimentally measured force-loading rates of individual molecular clutches and correctly predicts cell-adhesion behaviour for a range of substrate stiffnesses. The framework presented can be extended to study complex phenomena such as focal-adhesion maturation and cell-type-specific mechanosensing. The cytoskeleton and extracellular matrix are linked by molecular clutches that enable cells to sense their environment. Now it is shown that the elastic properties of the clutches underpin stiffness sensing.

力传递机构的弹性特性决定了细胞粘附的多稳态力学敏感行为
细胞可以感知环境并对其做出反应。这一过程的核心是分子离合器的形成,这是细胞外基质和肌动蛋白细胞骨架之间的动态联系,由整合素和衔接蛋白介导。尽管众所周知,分子-离合器组件之间的力依赖相互作用对于传感基底刚度至关重要,但非线性适配器-蛋白质弹性的影响却知之甚少。在这里,我们表明接头蛋白弹性和分子离合器与细胞外基质之间的局部相互作用是细胞感知底物刚度的关键。我们提出了一种半分析理论,整合了实验测量的适配器蛋白的力响应来描述细胞粘附感。我们建议分子离合器探测局部机械底物特性,并共同发挥差分作用,允许逆行肌动蛋白流动和底物运动以不同的速率发生,同时保持它们之间稳定的机械耦合。我们的模型再现了实验测量的单个分子离合器的力加载率,并正确预测了一系列衬底刚度的细胞粘附行为。所提出的框架可以扩展到研究复杂的现象,如局部粘附成熟和细胞类型特异性机械传感。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Physics
物理-物理:综合
CiteScore
30.40
自引率
2.00%
发文量
349
审稿时长
4-8 weeks
期刊介绍:
Nature Physics is dedicated to publishing top-tier original research in physics with a fair and rigorous review process. It provides high visibility and access to a broad readership, maintaining high standards in copy editing and production, ensuring rapid publication, and maintaining independence from academic societies and other vested interests.
The journal presents two main research paper formats: Letters and Articles. Alongside primary research, Nature Physics serves as a central source for valuable information within the physics community through Review Articles, News & Views, Research Highlights covering crucial developments across the physics literature, Commentaries, Book Reviews, and Correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


