Disruption of PRC1 components RING1A and RING1B promotes angiogenesis via relieving BMP4 repression
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Angiogenesis is crucial for tissue homeostasis and vascular regeneration following ischemia or injury. Epigenetic regulation has emerged as a key determinant of angiogenic gene expression. The Polycomb repressive complex 1 (PRC1) is a major epigenetic regulator that mediates gene silencing through monoubiquitylation of histone H2A at lysine 119 (H2AK119ub), a process primarily catalyzed by its core components RING1A and RING1B. However, the role of RING1A and RING1B in angiogenesis remains unclear.Objectives
In this study, we aimed to investigate the function and underlying mechanism of RING1A and RING1B in regulating endothelial cell functions and angiogenesis.Methods
We performed loss-of-function experiments using siRNAs targeting RING1A and RING1B in vitro and in vivo. Endothelial function was evaluated by tube formation, acetylated low-density lipoprotein (ac-LDL) uptake, nitric oxide production, proliferation, and migration. To identify downstream targets, we integrated RNA sequencing data from RING1A or RING1B knockdown endothelial cells with Cleavage Under Targets and Tagmentation profiling of RING1A, RING1B, and H2AK119ub. In vivo angiogenesis was examined using a Matrigel plug model and a corneal alkali-burn injury model in mice.Results
Knockdown of RING1A and RING1B significantly promoted tube formation, ac-LDL uptake, and nitric oxide production. Notably, only RING1A knockdown impaired endothelial proliferation and migration. Both RING1A and RING1B knockdown drastically promoted angiogenesis in vivo. Integrative analysis identified BMP4 as a direct transcriptional target of PRC1-mediated repression during angiogenesis.Conclusion
Our findings indicate that RING1A and RING1B play a repressive role in angiogenesis by epigenetically silencing BMP4 gene expression through H2AK119ub. Targeting PRC1-mediated repression may represent a novel therapeutic approach to promote angiogenesis in ischemic diseases.
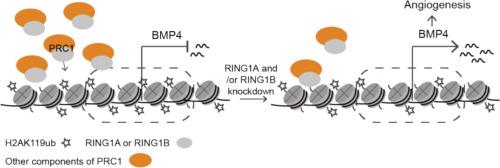
PRC1组分RING1A和RING1B的破坏通过缓解BMP4抑制促进血管生成
血管生成是缺血或损伤后组织稳态和血管再生的关键。表观遗传调控已成为血管生成基因表达的关键决定因素。Polycomb suppression complex 1 (PRC1)是一种主要的表观遗传调控因子,通过组蛋白H2A在赖氨酸119位点的单泛素化(H2AK119ub)介导基因沉默,这一过程主要由其核心组分RING1A和RING1B催化。然而,RING1A和RING1B在血管生成中的作用尚不清楚。目的本研究旨在探讨RING1A和RING1B在调节内皮细胞功能和血管生成中的作用及其机制。方法利用靶向RING1A和RING1B的sirna在体外和体内进行功能缺失实验。通过血管形成、乙酰化低密度脂蛋白(ac-LDL)摄取、一氧化氮生成、增殖和迁移来评估内皮功能。为了确定下游靶标,我们整合了RING1A或RING1B敲低内皮细胞的RNA测序数据,并结合了RING1A、RING1B和H2AK119ub的靶下切割和标记分析。采用基质塞模型和角膜碱烧伤模型观察小鼠体内血管生成情况。结果敲低RING1A和RING1B可显著促进小管形成、ac-LDL摄取和一氧化氮生成。值得注意的是,只有RING1A敲低会损害内皮细胞的增殖和迁移。RING1A和RING1B敲低均可显著促进体内血管生成。综合分析发现BMP4是血管生成过程中prc1介导的抑制的直接转录靶点。结论RING1A和RING1B通过H2AK119ub通过表观遗传沉默BMP4基因的表达,在血管生成中发挥抑制作用。靶向prc1介导的抑制可能是促进缺血性疾病血管生成的一种新的治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


