Evolving roles of MET as a therapeutic target in NSCLC and beyond
IF 82.2
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
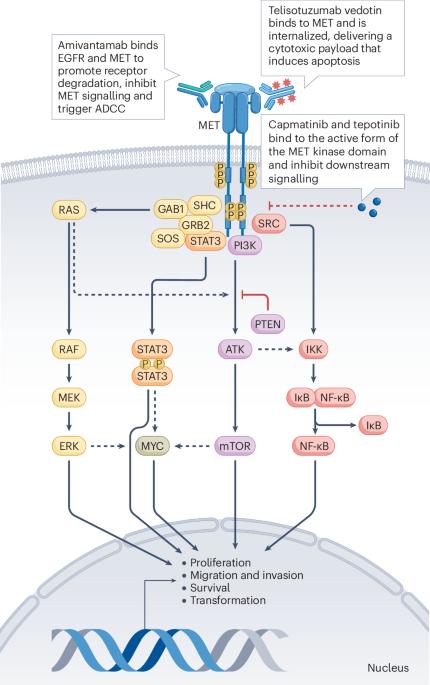
Alterations in the proto-oncogene MET are associated with tumour development, invasion and metastasis across various solid cancers. Therapeutically actionable MET alterations include MET exon 14 skipping (METex14) mutations, MET amplification and/or MET overexpression and MET fusions, which vary in incidence by tumour type. In contrast to rare de novo MET alterations, acquired MET amplification and/or MET overexpression is a relatively common phenomenon that is associated with distinct clinical implications and responses to treatment. METex14 is a distinct oncogenic driver mutation in non-small-cell lung cancer (NSCLC). To date, the MET tyrosine-kinase inhibitors (TKIs) capmatinib, tepotinib and savolitinib have been approved for the treatment of advanced-stage METex14-mutant NSCLC. However, the treatment paradigms for MET-altered solid tumours are rapidly evolving to include diverse MET-targeted agents. Emerging data support the role of MET TKIs, anti-MET antibodies and MET-directed antibody–drug conjugates (ADCs) as monotherapy or in combination with other therapies for NSCLC or other tumour types with MET amplification and/or overexpression. Indeed, in May 2025, the MET-directed ADC telisotuzumab vedotin was approved by the FDA for patients with previously treated advanced-stage nonsquamous NSCLC overexpressing MET (≥50% of tumour cells with 3+ staining on immunohistochemistry). Understanding the unique MET-related adverse events will be crucial when incorporating these agents into daily clinical practice. In this Review, we highlight the rationale for targeting MET alterations across various solid tumour types and provide a summary of the clinical efficacy and toxicity profiles of the approved and emerging MET-targeted TKIs, monoclonal or bispecific antibodies and ADCs. MET mutations, amplifications and fusions and MET overexpression are promising therapeutic targets across various cancers. In this Review, the authors summarize the prevalence, molecular diagnosis and prognostic implications of these alterations and discuss the clinical efficacy and toxicity profiles of diverse MET-targeted therapies, including tyrosine-kinase inhibitors, monoclonal or bispecific antibodies and antibody–drug conjugates.

MET作为非小细胞肺癌及其他疾病治疗靶点的演变作用
原癌基因MET的改变与各种实体癌的肿瘤发展、侵袭和转移有关。治疗上可操作的MET改变包括MET外显子14跳变(METex14)突变,MET扩增和/或MET过表达和MET融合,其发病率因肿瘤类型而异。与罕见的从头发生的MET改变相反,获得性MET扩增和/或MET过表达是一种相对常见的现象,与独特的临床意义和治疗反应相关。METex14是非小细胞肺癌(NSCLC)中一种独特的致癌驱动突变。迄今为止,MET酪氨酸激酶抑制剂(TKIs) capmatinib、tepoinib和savolitinib已被批准用于治疗晚期metex14突变体NSCLC。然而,met改变实体瘤的治疗模式正在迅速发展,包括多种met靶向药物。新出现的数据支持MET TKIs、抗MET抗体和MET定向抗体-药物偶联物(adc)作为单一疗法或与其他疗法联合治疗非小细胞肺癌或其他MET扩增和/或过表达的肿瘤类型的作用。事实上,在2025年5月,FDA批准了以MET为导向的ADC telisotuzumab vedotin,用于先前治疗过表达MET的晚期非鳞状NSCLC患者(≥50%的肿瘤细胞免疫组织化学染色为3+)。当将这些药物纳入日常临床实践时,了解met相关的独特不良事件将是至关重要的。在这篇综述中,我们强调了在各种实体肿瘤类型中靶向MET改变的基本原理,并概述了已批准的和新兴的MET靶向TKIs、单克隆或双特异性抗体和adc的临床疗效和毒性概况。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


