RVG29 and platelet membrane-modified pseudo-mitochondria for cascade targeting of cyclosporin a in traumatic brain injury
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Traumatic brain injury (TBI) is a major cause of mortality and morbidity worldwide. Cyclosporin A (CsA) is a promising drug for TBI, as it can protect neuronal mitochondria from dysfunction and apoptosis. However, CsA also has serious limitations, such as poor blood-brain barrier (BBB) penetration, non-selective distribution characteristic, and immunosuppressive effects. The latter is especially concerning, as TBI patients are highly susceptible to infections due to impaired immune function. Therefore, to realize the therapeutic potential of CsA for TBI, it is essential to achieve both efficient delivery of CsA to the injured brain and selective targeting of CsA to the neuronal mitochondria, while avoiding systemic immunosuppression. In this study, we borrowed from the fusion of mitochondria themselves and constructed pseudo-mitochondria based on the mitochondrial membrane. The pseudo-mitochondria successfully delivered the loaded substances to intracellular mitochondria by fusion with them. On this basis, we further achieved a cascade targeting delivery of CsA to injured neuron mitochondria in TBI through RVG29 and platelet membrane-modified pseudo-mitochondria, which significantly attenuated neuronal damage, neuroinflammation, and immunosuppression compared to CsA alone. Our study demonstrates the feasibility and potential of using RVG29 and platelet membrane-modified pseudo-mitochondria for cascade targeting of neuronal mitochondria in TBI therapy with CsA.
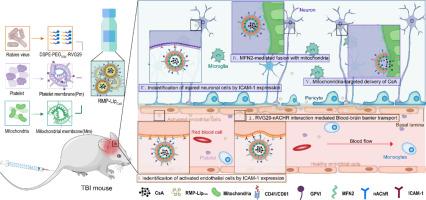

RVG29和血小板膜修饰伪线粒体在创伤性脑损伤环孢素a级联靶向中的作用
外伤性脑损伤(TBI)是世界范围内死亡率和发病率的主要原因之一。环孢素A (Cyclosporin A, CsA)具有保护神经元线粒体功能障碍和细胞凋亡的作用,是治疗创伤性脑损伤的重要药物。然而,CsA也有严重的局限性,如血脑屏障(BBB)渗透性差,非选择性分布特点,免疫抑制作用等。后者尤其令人担忧,因为创伤性脑损伤患者由于免疫功能受损而极易受到感染。因此,为了发挥CsA对TBI的治疗潜力,必须在避免全身免疫抑制的同时,实现CsA对损伤脑的有效递送和CsA对神经元线粒体的选择性靶向。在本研究中,我们借鉴线粒体自身的融合,在线粒体膜的基础上构建了伪线粒体。伪线粒体通过与细胞内线粒体融合,成功地将负载物质传递到细胞内线粒体。在此基础上,我们进一步实现了将CsA通过RVG29和血小板膜修饰的伪线粒体传递到TBI损伤神经元线粒体的级联,与单独CsA相比,CsA显著减轻了神经元损伤、神经炎症和免疫抑制。我们的研究证明了利用RVG29和血小板膜修饰的伪线粒体级联靶向神经元线粒体在CsA治疗TBI中的可行性和潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


