Microtubules coordinate mitochondria transport with myofibril morphogenesis during muscle development
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Muscle cells contain numerous energy-producing mitochondria and contractile myofibrils, whose myosin motors need ATP to generate force. Thus, myofibrils and mitochondria are in intimate contact in mature muscles. However, how their morphogenesis is coordinated during development remains largely unknown. Here, we used in vivo imaging to investigate myofibril and mitochondria network dynamics in developing Drosophila flight muscles. We found that mitochondria intercalate from the surface of actin bundles to their interior, and concomitantly, actin filaments condense to individual myofibrils. This ensures that mitochondria locate in proximity to every myofibril. Notably, antiparallel microtubules bundle with the assembling myofibrils, suggesting a key role in myofibril orientation. Indeed, microtubule severing affects myofibril orientation, whereas kinesin knockdown specifically blocks mitochondria intercalation. Importantly, mitochondria intercalation and their kinesin-dependent microtubule-based transport are conserved in mammalian muscle. Together, these data identify a key role for microtubules in coordinating mitochondria and myofibril morphogenesis to build functional muscles.
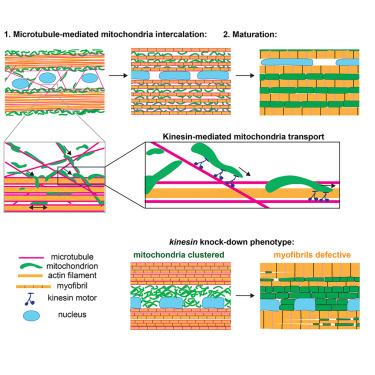
在肌肉发育过程中,微管协调线粒体运输和肌原纤维形态发生
肌肉细胞含有大量产生能量的线粒体和可收缩的肌原纤维,肌原纤维的肌球蛋白马达需要ATP来产生力量。因此,肌原纤维和线粒体在成熟肌肉中密切接触。然而,在发育过程中,它们的形态发生是如何协调的,在很大程度上仍然是未知的。在这里,我们使用体内成像来研究发育中的果蝇飞行肌肉中的肌原纤维和线粒体网络动力学。我们发现线粒体从肌动蛋白束的表面插入到其内部,同时,肌动蛋白丝浓缩成单个肌原纤维。这确保了线粒体位于每个肌原纤维附近。值得注意的是,反平行微管与组装的肌原纤维捆绑在一起,表明在肌原纤维定向中起关键作用。事实上,微管切断会影响肌原纤维的方向,而运动蛋白敲低则会特异性地阻断线粒体嵌入。重要的是,线粒体嵌入及其激酶依赖的微管转运在哺乳动物肌肉中是保守的。总之,这些数据确定了微管在协调线粒体和肌原纤维形态发生以建立功能性肌肉中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


