An aluminum-doped sodium manganese hexacyano ferrate cathode for high-rate safe Na-ion batteries†
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Aluminum (Al) doping in sodium manganese hexa-cyano-ferrate (NMHCF) stabilizes the cubic phase and suppresses multiphase formation. Doping reduces the particle size (∼0.35 μm), catalyzing the rate kinetics. Further Al-doping promotes aluminum-oxy-fluoride formation at the cathode electrolyte interphase for water in salt electrolytes, enabling safer, high-rate Na-ion battery performance.
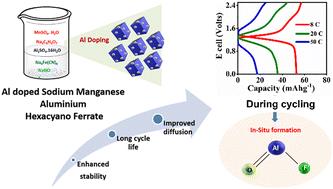
高速率安全钠离子电池用掺铝六氰高铁酸钠锰阴极
六氰高铁酸锰钠(NMHCF)阴极虽然具有很高的放电容量,但由于其不可避免的单斜态、立方态、四方态相变,其放电容量受到限制。研究表明,在NMHCF中掺入铝(Al)可以防止NMHCF的多相形成,并有利于立方相的形成。此外,与原始的NMHCF(~0.75µm)相比,Al掺杂可以形成更小的颗粒尺寸(~0.35µm)和更高的表面积,从而提高了速率动力学。除了稳定立方相外,Al的掺杂还促进了Al掺杂NMHCF (Al-NMHCF)阴极上氟化铝氧(AlOFx)阴极电解质间相(CEI)的形成,认为Al-NMHCF阴极为钠离子电池的安全、高倍率应用铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


