Evidence for trans-synaptic propagation of oligomeric tau in human progressive supranuclear palsy
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
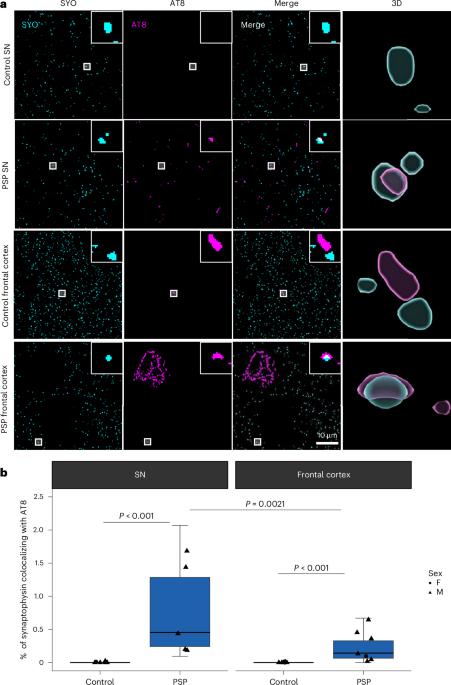
In the neurodegenerative disease progressive supranuclear palsy (PSP), tau pathology progresses through the brain in a stereotypical spatiotemporal pattern, and where tau pathology appears, synapses are lost. We tested the hypothesis that pathological tau contributes to synapse loss and may spread through the brain by moving from presynapses to postsynapses. Using postmortem PSP brain samples and a living human brain slice culture model, we observe pathological tau in synaptic pairs and evidence that oligomeric tau can enter live human postsynapses. Proteomics revealed increased clusterin in synapses in PSP, and super-resolution imaging showed clusterin colocalized with tau in synapses in close enough proximity to be binding partners, which may mediate tau spread. Accumulation of tau in synapses correlated with synapse loss, and synaptic engulfment by astrocytes was observed, suggesting that astrocytes contribute to synapse loss. Together, these data indicate that targeting synaptic tau is a promising approach to treat PSP. McGeachan et al. observe oligomeric tau in synapses from individuals with progressive supranuclear palsy and provide evidence that tau pathology may spread through the brain via synapses.

人类进行性核上性麻痹中低聚tau蛋白跨突触传播的证据
在神经退行性疾病进行性核上性麻痹(PSP)中,tau病理以一种典型的时空模式在大脑中发展,tau病理出现的地方突触丢失。我们测试了病理性tau有助于突触丢失的假设,并可能通过从突触前转移到突触后在大脑中传播。使用死后PSP脑样本和活体人脑切片培养模型,我们观察到突触对中的病理tau,并证明寡聚tau可以进入活体人脑突触后。蛋白质组学显示PSP突触中聚集蛋白增加,超分辨率成像显示聚集蛋白在突触中与tau蛋白共定位,距离足够近,可以成为tau蛋白的结合伙伴,这可能介导了tau蛋白的传播。突触中tau蛋白的积累与突触丢失相关,并且观察到星形胶质细胞对突触的吞噬,提示星形胶质细胞参与突触丢失。总之,这些数据表明,靶向突触tau是治疗PSP的一种有希望的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


