Synthetic Study on Bicyclic Pyranonaphthoquinone Natural Products: Construction of the Dioxabicyclo[3.3.1]nonene Motif
引用次数: 0
Abstract
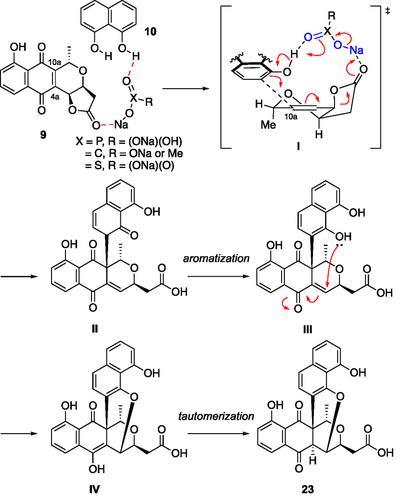
A concise method for the construction of dioxabicyclo[3.3.1]nonene framework has been developed. This structural motif has recently been identified in hybrid-type pyranonaphthoquinone-class natural products. The reaction proceeds in a stereoselective manner under mild conditions. In conjunction with this study, the scalable total syntheses of nanaomycins A and D have been achieved. Based on extensive screening of reaction conditions and nucleophiles, a plausible reaction mechanism is proposed.



双环吡萘醌类天然产物的合成研究:二恶双环[3.3.1]壬烯基序的构建
本文提出了一种构造二恶沙环[3.3.1]壬烯骨架的简便方法。这种结构基序最近在杂合型吡萘醌类天然产物中被发现。反应在温和条件下以立体选择性的方式进行。结合本研究,实现了纳米霉素A和D的规模化全合成。在广泛筛选反应条件和亲核试剂的基础上,提出了一个合理的反应机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


