Structural and site-specific characterization of distinctive N-glycans with heavy fucosylation in human semen
IF 6.5
Q1 CHEMISTRY, APPLIED
Carbohydrate Polymer Technologies and Applications
Pub Date : 2025-07-09
DOI:10.1016/j.carpta.2025.100941
引用次数: 0
Abstract
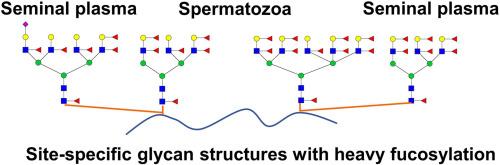
Heavy fucosylation (fucose residues ≥ 6 per glycan) has been previously reported in human semen with unclear precise site-specific glycan structures. In current study, we characterized heavily fucosylated glycoproteins as a distinctive feature of human spermatozoa (HS) and seminal plasma (HSP), with a precise definition of glycan structural features at the glycosite-specific level. There were 49 unique heavily fucosylated intact glycopeptides (IGPs) at 15 N-glycosites from 12 glycoproteins identified in HS, and 188 unique heavily fucosylated IGPs at 58 N-glycosites from 37 glycoproteins in HSP. Among these heavily fucosylated glycoproteins, 10 were shared in HS and HSP, 2 were detected only in HS and 17 only in HSP. Almost all heavily fucosylated glycans were complex N-glycans with core fucosylation and Lewis antennary, among which CLU were glycosylated by ten and nine fucoses per glycan in HS and HSP, respectively. Moreover, these heavily fucosylated glycans varied from tri- to hexa-antennas. Notably, the N-glycan structures on shared heavily fucosylated glycoproteins were more complex in HSP than in HS. These heavily fucosylated glycoproteins identified in human semen represent a valuable and distinctive resource for glycopeptide studies, offering significant potential for advancing glycoproteomic methodologies and clinical research into male infertility.
人类精液中高度聚焦的n -聚糖的结构和位点特异性表征
重度聚焦化(每个聚糖的聚焦残基≥6个)在人类精液中有报道,但不清楚精确的位点特异性聚糖结构。在目前的研究中,我们将高度聚焦的糖蛋白描述为人类精子(HS)和精浆(HSP)的一个独特特征,并在糖位点特异性水平上精确定义了聚糖结构特征。在HS中鉴定的12种糖蛋白的15个n -糖位点上有49个独特的高度聚焦的完整糖肽(IGPs),在HSP中鉴定的37种糖蛋白的58个n -糖位点上有188个独特的高度聚焦的完整糖肽(IGPs)。在这些高度集中的糖蛋白中,10个在HS和HSP中共享,2个仅在HS中检测到,17个仅在HSP中检测到。几乎所有重聚焦的聚糖都是具有核心聚焦和Lewis天线的复合n -聚糖,其中CLU在HS和HSP中分别被10个和9个焦点糖基化。此外,这些高度聚焦的聚糖从三天线到六天线不等。值得注意的是,在共享的高度集中的糖蛋白上的n -聚糖结构在HSP中比在HS中更复杂。这些在人类精液中发现的高度集中的糖蛋白代表了糖肽研究的宝贵和独特的资源,为推进糖蛋白组学方法和男性不育的临床研究提供了巨大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


