Baicalein reduces cardiac inflammatory infiltration in EAM mice by blocking the CCL2-CCR2 signaling axis through its binding with TNF-α and CCR2
IF 4.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Myocarditis refers to localized or diffuse inflammatory lesions of the myocardium. Experimental autoimmune myocarditis (EAM) in mice is commonly utilized as an animal model for studying the pathogenesis of myocarditis. Baicalein (BAI), the main active component extracted from Scutellaria baicalensis root, has been proven to possess diverse effects such as anti-inflammatory, anti-tumor, and antioxidant activities. However, further investigation is warranted to elucidate the mechanism of action underlying BAI's efficacy in EAM. The aim of this study is to the potential of BAI in combination with TNF-α to downregulate the TNF-α/TNFR1-AP-1 signaling pathway. Furthermore, we will explore whether BAI exhibits an inhibitory effect on the CCL2/CCR2-ROCK1 signaling pathway. In this study, we employed the EAM animal model to investigate the inhibitory effect of BAI on macrophage and Th1 cell chemotaxis towards cardiac tissue in EAM mice. Techniques such as HE staining, immunofluorescence, and other methods were utilized for assessment. Additionally, computer-simulated molecular docking, Streptavidin pull-down, and co-immunoprecipitation experiments were conducted to explore the potential binding of BAI with TNF-α and CCR2. Furthermore, real-time quantitative polymerase chain reaction (qPCR), western blotting, and flow cytometry were employed to elucidate the impact of BAI on the TNF-α/TNFR1-CCL2/CCR2 signaling pathway. BAI suppressed the expression of chemokine CCL2 in EAM mouse myocardial tissue and attenuated the infiltration of macrophages and Th1 cells. In vitro, BAI exhibited binding affinity to TNF-α, leading to downregulation of the TNF-α/TNFR1-AP-1 signaling pathway and subsequent inhibition of CCL2 secretion by macrophages and vascular endothelial cells. Additionally, BAI demonstrated binding capability to CCR2, resulting in downregulation of the CCL2/CCR2-ROCK1 pathway and consequent inhibition of chemotactic migration of macrophages and Th1 cells. This study demonstrates that BAI can downregulate the secretion of CCL2 and the CCL2/CCR2-ROCK1 signaling pathway by binding with TNF-α and CCR2, thereby inhibiting the migration of macrophages and Th1 cells to the lesion site, thus alleviating the inflammation severity in EAM.
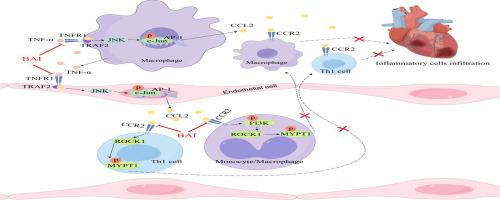
黄芩素通过与TNF-α和CCR2结合,阻断CCL2-CCR2信号轴,减少EAM小鼠心脏炎症浸润。
背景:心肌炎是指心肌的局部或弥漫性炎性病变。小鼠实验性自身免疫性心肌炎(EAM)是研究心肌炎发病机制的常用动物模型。黄芩素(Baicalein, BAI)是从黄芩根中提取的主要活性成分,具有抗炎、抗肿瘤、抗氧化等多种作用。然而,需要进一步的研究来阐明BAI在EAM中的作用机制。目的:本研究旨在探讨BAI联合TNF-α下调TNF-α/TNFR1-AP-1信号通路的潜力。此外,我们将探索BAI是否对CCL2/CCR2-ROCK1信号通路具有抑制作用。研究设计和方法:本研究采用EAM动物模型,研究BAI对EAM小鼠巨噬细胞和Th1细胞向心脏组织趋化的抑制作用。利用HE染色、免疫荧光等技术进行评估。此外,通过计算机模拟的分子对接、链亲和素下拉和共免疫沉淀实验来探索BAI与TNF-α和CCR2的潜在结合。此外,采用实时定量聚合酶链反应(qPCR)、western blotting和流式细胞术分析BAI对TNF-α/TNFR1-CCL2/CCR2信号通路的影响。结果:BAI抑制EAM小鼠心肌组织趋化因子CCL2的表达,减轻巨噬细胞和Th1细胞的浸润。在体外,BAI与TNF-α表现出结合亲和力,导致TNF-α/TNFR1-AP-1信号通路下调,从而抑制巨噬细胞和血管内皮细胞分泌CCL2。此外,BAI显示出与CCR2的结合能力,导致CCL2/CCR2- rock1通路下调,从而抑制巨噬细胞和Th1细胞的趋化迁移。结论:本研究表明BAI可通过与TNF-α和CCR2结合,下调CCL2分泌及CCL2/CCR2- rock1信号通路,从而抑制巨噬细胞和Th1细胞向病变部位的迁移,从而减轻EAM炎症的严重程度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


