Direct interactions between the human insula and hippocampus during memory encoding
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
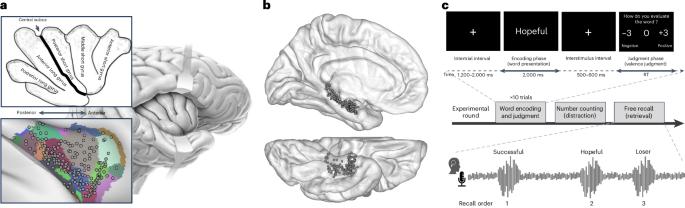
The hippocampus is critical for encoding episodic memories, but how it interacts with cortical regions during this process remains unclear. In this study, 16 participants with implanted electrodes in the insula (217 sites) and hippocampus (131 sites) viewed emotionally valenced words and attempted to recall them. During encoding, one subset of insular neuronal populations showed changes in aperiodic activity that predicted successful recall. These insular changes followed hippocampal theta but preceded hippocampal ripples. Another subset of insular sites responded to word valence, unrelated to memory performance. Direct electrical stimulation of memory-related insular sites evoked early responses in the ipsilateral hippocampus, whereas stimulation of valence-related sites did not. Conversely, stimulating hippocampal sites produced slow, variable signals across all insular sites, suggesting asymmetric communication between the hippocampus and the insula. These findings provide a glimpse of mesoscale hippocampal interactions with functionally selective neuronal populations within a given cortical structure. The hippocampus and insula communicate when processing emotional memories. Discrete sites in the human insular cortex showed changes that predicted later memory recall, while others responded to emotional content.

人类脑岛与海马体在记忆编码过程中的直接相互作用
海马体对情景记忆的编码至关重要,但在这个过程中它是如何与皮层区域相互作用的还不清楚。在这项研究中,16名参与者在脑岛(217个位点)和海马体(131个位点)植入电极,观看有情感价值的单词,并试图回忆它们。在编码过程中,岛状神经元群的一个子集显示出预测成功回忆的非周期性活动的变化。这些岛屿的变化发生在海马体θ波之后,但发生在海马体波纹之前。另一部分岛状部位对单词效价有反应,与记忆表现无关。直接电刺激与记忆相关的岛岛部位诱发了同侧海马的早期反应,而对价相关部位的刺激则没有。相反,刺激海马体部位在所有岛岛部位产生缓慢、可变的信号,表明海马体和岛岛之间的通信不对称。这些发现提供了中尺度海马与特定皮质结构内功能选择性神经元群相互作用的一瞥。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


