Unveiling the reconstruction of copper bimetallic catalysts during CO2 electroreduction
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Efficient electrocatalysts should provide optimal binding sites for intermediates under operating conditions. Atomic rearrangements in catalysts during electrochemical CO2 reduction reaction (CO2RR) alter the original structures of active sites. Here we report a general principle for understanding and predicting the reconstruction of Cu bimetallic catalysts during CO2RR in terms of selective dissolution–redeposition. We categorize the reconstruction trends of Cu bound to a secondary metal (X, where X = Ag, Fe, Zn or Pd) according to the oxophilicity and miscibility of Cu and X. Cross-sectional microscopy analysis of gas diffusion electrodes reveals that the surface states of reconstructed Cu–X are determined by atomic miscibility. We find that CO2RR intermediates alter elemental preferences for dissolution, shifting them away from oxophilicity-governed behaviour and leading to selective Cu dissolution–redeposition in Cu–X. This reconstruction affects spillover in CO2RR, controlling the selectivities of ethylene/ethanol and C1/C2 products. We also develop a methodology for the control of reconstruction dynamics. Our findings provide insights into designing catalysts that undergo reconstruction during electrolysis. Reconstruction of Cu catalysts under electrochemical CO2 reduction conditions is a well-reported phenomenon. Here the reconstruction of bimetallic Cu–X catalysts is investigated to reveal the roles of atomic miscibility and intermediate binding on surface reconstruction and the resulting effects on product selectivity.
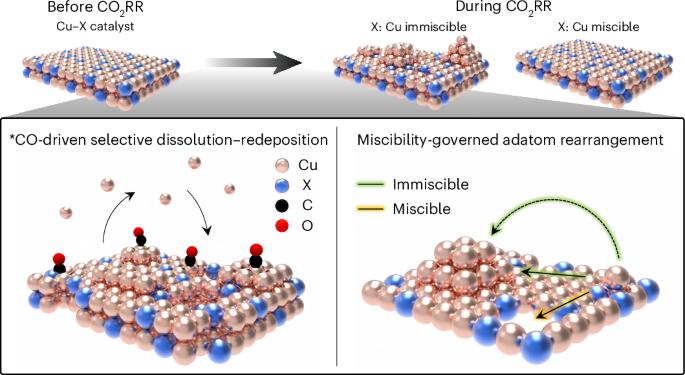

揭示CO2电还原过程中铜双金属催化剂的重构
高效的电催化剂应该在操作条件下为中间体提供最佳的结合位点。电化学CO2还原反应(CO2RR)中催化剂的原子重排改变了活性位点的原始结构。在这里,我们报告了从选择性溶解-再沉积的角度来理解和预测Cu双金属催化剂在CO2RR过程中的重构的一般原则。我们根据Cu和X的亲氧性和混溶性对Cu与次级金属(X,其中X = Ag, Fe, Zn或Pd)结合的重建趋势进行了分类。气体扩散电极的横截面显微镜分析表明,重建的Cu - X的表面状态是由原子混溶性决定的。我们发现,CO2RR中间体改变了元素对溶解的偏好,使它们远离亲氧控制的行为,并导致Cu在Cu - x中的选择性溶解-再沉积。这种重构影响了CO2RR的溢出,控制了乙烯/乙醇和C1/C2产物的选择性。我们还开发了一种方法来控制重建动力学。我们的发现为设计在电解过程中进行重构的催化剂提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


