Exposure to chlorothalonil in zebrafish: modulation and biotransformation potential of two cytosolic GST isoforms
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Glutathione S-transferases (GSTs) are enzymes involved in the Phase II biotransformation of xenobiotics, including chlorothalonil (CLT), an organochlorine biocide used in agriculture, and antifouling paints. However, GSTs comprise a family of related enzymes and specific isoforms that are preferentially involved in CLT metabolism have not yet been identified. Thus, the objective of this study was to identify whether the two cytosolic GST isoforms expressed in zebrafish gills (GSTP and GSTA) were modulated by CLT exposure and their potential to participate in CLT metabolism in this species. Initially, the activity of both GST isoforms was evaluated in zebrafish gills after chlorothalonil exposure (to 0.1 and 10 μg/L) for 4 and 7 days. The highest CLT concentration tested (10 μg/L) significantly increased GSTP activity after 4 days of exposure and the activity of GSTA after 7 days of exposure. The mRNA levels of the gstp (gstp1 and gstp1) and gsta (gsta1 and gsta2) isoforms were also evaluated in fish gills after exposure to the same CLT concentration, but no significant alteration was observed. Finally, in silico analysis strongly suggests that CLT could interact with the active site of both GST isoforms with good binding affinity, following a spontaneous thermodynamic process (ΔG < 0 kcal/mol). The approach employed here indicated that both GST isoforms could be involved in the biotransformation of CLT in the gills of D. rerio while protecting the fish against the deleterious effects of contaminants.
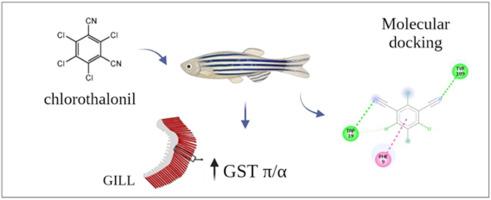
斑马鱼暴露于百菌清:两种细胞质GST亚型的调节和生物转化潜力。
谷胱甘肽s -转移酶(GSTs)是一种参与外源性生物转化的酶,包括百菌清(CLT),一种用于农业的有机氯杀菌剂,以及防污涂料。然而,gst包括一个优先参与CLT代谢的相关酶家族和特定同种异构体尚未被确定。因此,本研究的目的是确定斑马鱼鳃中表达的两种胞质GST亚型(GSTP和GSTA)是否受到CLT暴露的调节,以及它们参与该物种CLT代谢的潜力。首先,在暴露于(0.1和10 μg/L)百菌清4天和7天后,在斑马鱼鳃中评估了两种GST亚型的活性。最高CLT浓度(10 μg/L)可显著提高暴露4 d后GSTP活性和暴露7 d后GSTA活性。同样浓度的CLT对鱼鳃中gstp (gstp1和gstp1)和gsta (gsta1和gsta2)亚型的mRNA水平也进行了评估,但未观察到显著变化。最后,硅分析强烈表明,CLT可以通过自发热力学过程(ΔG < 0 kcal/mol)与两种GST同工异构体的活性位点相互作用,并具有良好的结合亲和力。本文采用的方法表明,两种GST异构体都可能参与鱼鳃中CLT的生物转化,同时保护鱼免受污染物的有害影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


