Metallothionein CgMTIII is involved in zinc binding and accumulation in the Pacific oyster Crassostrea gigas
IF 1.8
3区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology
Pub Date : 2025-07-10
DOI:10.1016/j.cbpb.2025.111128
引用次数: 0
Abstract
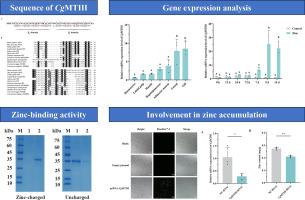
Metallothioneins (MTs) are a family of small, metal-binding proteins that play a pivotal role in metal storage and detoxification. The Pacific oyster Crassostrea gigas is known for its exceptionally high zinc content, and investigating the role of MTs in oyster zinc metabolism will help elucidate the mechanisms underlying its zinc accumulation. In this study, a MT homolog CgMTIII was cloned from C. gigas to investigate its function in zinc binding and accumulation. The transcripts of CgMTIII were distributed in all examined oyster tissues including gill, mantle, adductor muscle, hepatopancreas, gonad, labial palp and haemocytes, with the highest expression in the gill and the lowest in the haemocytes. Immobilized metal ion affinity chromatography (IMAC) demonstrated that the recombinant CgMTIII protein exhibited high retention on the zinc-charged column, confirming its zinc-binding activity. Overexpression of CgMTIII in HEK293T cells led to a 2.34-fold increase in intracellular zinc content after 24 h of exposure to 100 μM zinc, compared to the control group. Moreover, knockdown of CgMTIII through RNA interference resulted in a 24% reduction in zinc content in the gill tissue of oysters. Collectively, CgMTIII exhibited zinc-binding activity and contributed significantly to zinc accumulation in C. gigas. These findings deepen our understanding of the zinc-enrichment mechanism in oysters and provide a theoretical foundation for breeding high‑zinc oyster varieties.
金属硫蛋白CgMTIII参与了太平洋牡蛎长牡蛎中锌的结合和积累。
金属硫蛋白(MTs)是一类小的金属结合蛋白,在金属储存和解毒中起关键作用。太平洋牡蛎长牡蛎以其极高的锌含量而闻名,研究MTs在牡蛎锌代谢中的作用将有助于阐明其锌积累的机制。本研究从C. gigas中克隆了一个MT同源基因CgMTIII,以研究其在锌结合和积累中的功能。CgMTIII转录本分布在牡蛎的鳃、套、内收肌、肝胰腺、性腺、唇瓣和血细胞中,其中鳃中表达量最高,血细胞中表达量最低。固定化金属离子亲和层析(IMAC)表明,重组CgMTIII蛋白在带锌柱上具有较高的保留率,证实了其与锌的结合活性。与对照组相比,暴露于100 μM锌24 h后,HEK293T细胞中CgMTIII的过表达导致细胞内锌含量增加2.34倍。此外,通过RNA干扰敲除CgMTIII导致牡蛎鳃组织中锌含量降低24% %。综上所述,CgMTIII表现出锌结合活性,并对C. gigas中锌的积累有显著贡献。这些发现加深了我们对牡蛎锌富集机制的认识,为培育高锌牡蛎品种提供了理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.60
自引率
4.50%
发文量
77
审稿时长
22 days
期刊介绍:
Comparative Biochemistry & Physiology (CBP) publishes papers in comparative, environmental and evolutionary physiology.
Part B: Biochemical and Molecular Biology (CBPB), focuses on biochemical physiology, primarily bioenergetics/energy metabolism, cell biology, cellular stress responses, enzymology, intermediary metabolism, macromolecular structure and function, gene regulation, evolutionary genetics. Most studies focus on biochemical or molecular analyses that have clear ramifications for physiological processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


