miR-363-5p- and IL-34-mediated modulation of pacemaker channel, HCN4, on iPSC-CM: Translation into the human sinus node microenvironment
IF 5.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
The human sinus node (SN) contains cardiac fibroblasts and resident macrophages, with microRNAs (miRNAs) and interleukins as regulators of SN function. However, the mechanisms by which they influence heart rate remain unclear.
Objective
This study aimed to investigate the SN microenvironment, encompassing miRNAs, interleukins, macrophages, and fibroblasts and modulating induced pluripotent stem cell–derived cardiomyocytes (iPSC-CMs) and hence beating rate.
Methods
Multiomics analysis was conducted to compare human SN with the right atria. Human iPSC-CMs were cocultured with M2-type macrophages (M2s) and fibroblasts. Mechanistic studies involved the application of interleukin 34 (IL-34) and the silencing or overexpression of colony-stimulating factor 1 receptor and signal transducer and activator of transcription 3. Messenger RNA–miRNA interactions were predicted using the Ingenuity Pathway Analysis. miR-363-5p was applied to 3 cell lines and a coculture system and, along with IL-34, was tested in human serum samples.
Results
M2 and fibroblast markers were identified. Coculturing iPSC-CMs with M2s induced a more nodal-like phenotype. Elevated IL-34 in the coculture medium suggested that M2s may secrete IL-34, driving these nodal-like features. IL-34 promoted a nodal-like phenotype in iPSC-CMs by upregulating hyperpolarization-activated cyclic nucleotide-gated channel subtype 4 expression probably through colony-stimulating factor 1 receptor/signal transducer and activator of transcription 3 signaling. Three key miRNAs were identified, with miR-363-5p inhibiting the differentiation of macrophages into the M2 phenotype. The nodal-like shift of iPSC-CMs in the coculture system was reversed by miR-363-5p. Although miR-363-5p (together with miR-486-3p) was highly expressed, IL-34 was reduced in aged individuals with SN dysfunction.
Conclusion
M2s contribute to the SN microenvironment by secreting IL-34 and thus enhance SN function by upregulating hyperpolarization-activated cyclic nucleotide-gated channel subtype 4. Elevated miR-363-5p, seen in age-related SN dysfunction, may reverse these effects.
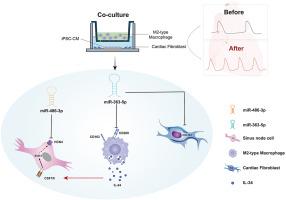
miR-363-5p和IL-34介导的起搏器通道HCN4对iPSC-CM的调节转化为人类窦房结微环境。
背景:人窦房结(SN)含有心脏成纤维细胞和常驻巨噬细胞,微rna (miRNAs)和白细胞介素作为窦房结功能的调节因子。然而,它们如何影响心率(HR)的机制尚不清楚。目的:本研究旨在研究SN微环境,包括mirna、白细胞介素、巨噬细胞和成纤维细胞,并调节iPSC-CMs的跳动速率。方法:采用多组学方法对人右心房(RA)与SN进行比较。人ipsc来源的心肌细胞(iPSC-CMs)与m2型巨噬细胞(M2s)和成纤维细胞共培养。机制研究涉及IL-34的应用以及CSF1R和STAT3的沉默或过表达。利用独创性途径分析(Ingenuity Pathway Analysis, IPA)预测mRNA-miRNA相互作用。将miR-363-5p应用于三个细胞系和共培养系统,并与IL-34一起在人血清样本中进行测试。结果:鉴定出M2s和成纤维细胞标记物。iPSC-CMs与M2s共培养诱导出更类似于结节的表型。共培养培养基中IL-34升高提示M2s可能分泌IL-34,驱动这些淋巴结样特征。IL-34可能通过CSF1R/STAT3信号通路上调HCN4表达,从而促进iPSC-CMs的结节样表型。鉴定出三个关键mirna,其中miR-363-5p抑制巨噬细胞向M2表型分化。共培养系统中iPSC-CMs的结节样转移被miR-363-5p逆转。虽然miR-363-5p(连同miR-486-3p)高表达,但IL-34在老年SND患者中降低。结论:M2巨噬细胞通过分泌IL-34参与SN微环境,通过上调HCN4增强SN功能。在与年龄相关的SND中,miR-363-5p的升高可能会逆转这些作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Heart rhythm
医学-心血管系统
CiteScore
10.50
自引率
5.50%
发文量
1465
审稿时长
24 days
期刊介绍:
HeartRhythm, the official Journal of the Heart Rhythm Society and the Cardiac Electrophysiology Society, is a unique journal for fundamental discovery and clinical applicability.
HeartRhythm integrates the entire cardiac electrophysiology (EP) community from basic and clinical academic researchers, private practitioners, engineers, allied professionals, industry, and trainees, all of whom are vital and interdependent members of our EP community.
The Heart Rhythm Society is the international leader in science, education, and advocacy for cardiac arrhythmia professionals and patients, and the primary information resource on heart rhythm disorders. Its mission is to improve the care of patients by promoting research, education, and optimal health care policies and standards.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


